Related Research Articles

In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

Cyclin-dependent kinases (CDKs) are the families of protein kinases first discovered for their role in regulating the cell cycle. They are also involved in regulating transcription, mRNA processing, and the differentiation of nerve cells. They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved. In fact, yeast cells can proliferate normally when their CDK gene has been replaced with the homologous human gene. CDKs are relatively small proteins, with molecular weights ranging from 34 to 40 kDa, and contain little more than the kinase domain. By definition, a CDK binds a regulatory protein called a cyclin. Without cyclin, CDK has little kinase activity; only the cyclin-CDK complex is an active kinase but its activity can be typically further modulated by phosphorylation and other binding proteins, like p27. CDKs phosphorylate their substrates on serines and threonines, so they are serine-threonine kinases. The consensus sequence for the phosphorylation site in the amino acid sequence of a CDK substrate is [S/T*]PX[K/R], where S/T* is the phosphorylated serine or threonine, P is proline, X is any amino acid, K is lysine, and R is arginine.
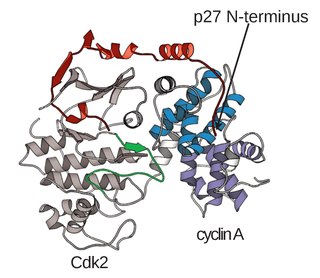
A cyclin-dependent kinase complex is a protein complex formed by the association of an inactive catalytic subunit of a protein kinase, cyclin-dependent kinase (CDK), with a regulatory subunit, cyclin. Once cyclin-dependent kinases bind to cyclin, the formed complex is in an activated state. Substrate specificity of the activated complex is mainly established by the associated cyclin within the complex. Activity of CDKCs is controlled by phosphorylation of target proteins, as well as binding of inhibitory proteins.
Maturation-promoting factor (abbreviated MPF, also called mitosis-promoting factor or M-Phase-promoting factor) is the cyclin-Cdk complex that was discovered first in frog eggs. It stimulates the mitotic and meiotic phases of the cell cycle. MPF promotes the entrance into mitosis (the M phase) from the G2 phase by phosphorylating multiple proteins needed during mitosis. MPF is activated at the end of G2 by a phosphatase, which removes an inhibitory phosphate group added earlier.

The restriction point (R), also known as the Start or G1/S checkpoint, is a cell cycle checkpoint in the G1 phase of the animal cell cycle at which the cell becomes "committed" to the cell cycle, and after which extracellular signals are no longer required to stimulate proliferation. The defining biochemical feature of the restriction point is the activation of G1/S- and S-phase cyclin-CDK complexes, which in turn phosphorylate proteins that initiate DNA replication, centrosome duplication, and other early cell cycle events. It is one of three main cell cycle checkpoints, the other two being the G2-M DNA damage checkpoint and the spindle checkpoint.

Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, with progression through the various phases of the cell cycle occurring only when favorable conditions are met. There are many checkpoints in the cell cycle, but the three major ones are: the G1 checkpoint, also known as the Start or restriction checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint. Progression through these checkpoints is largely determined by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell cycle to control the specific events that occur therein.
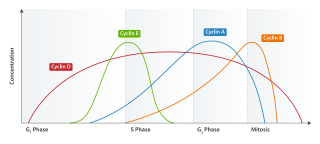
Cyclin E is a member of the cyclin family.

CDK-activating kinase (CAK) activates the cyclin-CDK complex by phosphorylating threonine residue 160 in the CDK activation loop. CAK itself is a member of the Cdk family and functions as a positive regulator of Cdk1, Cdk2, Cdk4, and Cdk6.

Cyclin D is a member of the cyclin protein family that is involved in regulating cell cycle progression. The synthesis of cyclin D is initiated during G1 and drives the G1/S phase transition. Cyclin D protein is anywhere from 155 to 477 amino acids in length.

Cyclin-dependent kinase 2, also known as cell division protein kinase 2, or Cdk2, is an enzyme that in humans is encoded by the CDK2 gene. The protein encoded by this gene is a member of the cyclin-dependent kinase family of Ser/Thr protein kinases. This protein kinase is highly similar to the gene products of S. cerevisiae cdc28, and S. pombe cdc2, also known as Cdk1 in humans. It is a catalytic subunit of the cyclin-dependent kinase complex, whose activity is restricted to the G1-S phase of the cell cycle, where cells make proteins necessary for mitosis and replicate their DNA. This protein associates with and is regulated by the regulatory subunits of the complex including cyclin E or A. Cyclin E binds G1 phase Cdk2, which is required for the transition from G1 to S phase while binding with Cyclin A is required to progress through the S phase. Its activity is also regulated by phosphorylation. Multiple alternatively spliced variants and multiple transcription initiation sites of this gene have been reported. The role of this protein in G1-S transition has been recently questioned as cells lacking Cdk2 are reported to have no problem during this transition.

Cell division protein kinase 6 (CDK6) is an enzyme encoded by the CDK6 gene. It is regulated by cyclins, more specifically by Cyclin D proteins and Cyclin-dependent kinase inhibitor proteins. The protein encoded by this gene is a member of the cyclin-dependent kinase, (CDK) family, which includes CDK4. CDK family members are highly similar to the gene products of Saccharomyces cerevisiae cdc28, and Schizosaccharomyces pombe cdc2, and are known to be important regulators of cell cycle progression in the point of regulation named R or restriction point.
Transcription factor II H (TFIIH) is an important protein complex, having roles in transcription of various protein-coding genes and DNA nucleotide excision repair (NER) pathways. TFIIH first came to light in 1989 when general transcription factor-δ or basic transcription factor 2 was characterized as an indispensable transcription factor in vitro. This factor was also isolated from yeast and finally named TFIIH in 1992.
The Cyclin D/Cdk4 complex is a multi-protein structure consisting of the proteins Cyclin D and cyclin-dependent kinase 4, or Cdk4, a serine-threonine kinase. This complex is one of many cyclin/cyclin-dependent kinase complexes that are the "hearts of the cell-cycle control system" and govern the cell cycle and its progression. As its name would suggest, the cyclin-dependent kinase is only active and able to phosphorylate its substrates when it is bound by the corresponding cyclin. The Cyclin D/Cdk4 complex is integral for the progression of the cell from the Growth 1 phase to the Synthesis phase of the cell cycle, for the Start or G1/S checkpoint.

Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine protein kinase, and is a key player in cell cycle regulation. It has been highly studied in the budding yeast S. cerevisiae, and the fission yeast S. pombe, where it is encoded by genes cdc28 and cdc2, respectively. With its cyclin partners, Cdk1 forms complexes that phosphorylate a variety of target substrates ; phosphorylation of these proteins leads to cell cycle progression.

Cyclin-dependent kinase 7, or cell division protein kinase 7, is an enzyme that in humans is encoded by the CDK7 gene.

CDK-activating kinase assembly factor MAT1 is an enzyme that in humans is encoded by the MNAT1 gene.

Cyclin-H is a protein that in humans is encoded by the CCNH gene.

General transcription factor IIH subunit 1 is a protein that in humans is encoded by the GTF2H1 gene.
RNA polymerase II holoenzyme is a form of eukaryotic RNA polymerase II that is recruited to the promoters of protein-coding genes in living cells. It consists of RNA polymerase II, a subset of general transcription factors, and regulatory proteins known as SRB proteins.
A series of biochemical switches control transitions between and within the various phases of the cell cycle. The cell cycle is a series of complex, ordered, sequential events that control how a single cell divides into two cells, and involves several different phases. The phases include the G1 and G2 phases, DNA replication or S phase, and the actual process of cell division, mitosis or M phase. During the M phase, the chromosomes separate and cytokinesis occurs.
References
- ↑ Morgan DO. (2007). The Cell Cycle: Principles of Control. New Science Press Ltd: London, UK
- ↑ Harper, J. W., Elledge, S. J., Keyomarski, K., Dynlacht, B., Tsai, L.-H., Zhang, P., Dobrowolski, S., Bai, C., Connell-Crowley, L., Swindell, E. et al. (1995). Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6, 387-400
- ↑ Lolli, G., Lowe, E. D., Brown, N. R. and Johnson, L. N. (2004). The crystal structure of human CDK7 and its protein recognition properties. Structure 12, 2067-2079
- ↑ Akoulitchev, S. and Reinberg, D. (1998). The molecular mechanism of mitotic inhibition of TFIIH is mediated by phosphorylation of CDK7. Genes Dev. 12, 3541-3550
- 1 2 Patel, Shetal A., and M. Celeste Simon. "Functional analysis of the CDK7· cyclin H· Mat1 complex in mouse embryonic stem cells and embryos." Journal of Biological Chemistry 285.20 (2010): 15587-15598.
- 1 2 3 Lolli, Graziano, and Louise N. Johnson. "CAK—cyclin-dependent activating kinase: a key kinase in cell cycle control and a target for drugs?" Cell cycle 4.4 (2005): 565-570.
- 1 2 Larochelle, S et al. “T-loop phosphorylation stabilizes the CDK7-cyclin H-MAT1 complex in vivo and regulates its CTD kinase activity.” The EMBO Journal vol. 20,14 (2001): 3749-59. doi:10.1093/emboj/20.14.3749
- ↑ Morgan DO: Principles of CDK regulation. Nature 1995, 374:131-134
- ↑ Solomon MJ: The function(s) of CAK, the p34cdc2 activating kinase. Trends Biochem Sci 1994,19:496-500
- ↑ Connell-Cowley L, Solomon MJ, Wei N, Harper JW: Phosphorylation independent activation of human cyclindependent kinase 2 by cyclin A in vitro. Mol Biol Cell 1993, 4:79-92
- ↑ Matsuoka M, Kate JY, Fisher RP, Mor of cyclin-dependent kinase 4 (cdk4 by B mouse M015-an associated klnase. Mol Cell Biol 1994, 14:7265-7275.
- ↑ Fisher, Robert P. "Secrets of a double agent: CDK7 in cell-cycle control and transcription." Journal of cell science 118.22 (2005): 5171-5180.
- ↑ Schachter, Miriam Merzel, et al. "A Cdk7-Cdk4 T-loop phosphorylation cascade promotes G1 progression." Molecular cell 50.2 (2013): 250-260.
- ↑ Roy R, Adamczewski JP, Seroz T, Vermeulen W, Tassan JP, Schaeffer L, Nigg EA, Hoejimakers JHJ, Egly JM: The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell 1994, 79:1093-1101
- ↑ Seroz T, Hwang JR, Moncollin V, Egly JM: TFIIH: a link between transcription, DNA repair and cell cycle regulation. Gun Opin Gener Dev 1995, 5:217-221
- ↑ Dahmus ME: The role of multisite phosphorylatlon in the regulation of RNA polymerase II activity. Prog Nucleic Acid Res Mol Biol 1994, 48: 143-179
- ↑ Shiekhattar R, Mermelstein F, Fisher R, Drapkin R, Dynlacht B, Wessling HC, Morgan DO, Reinberg D: Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 1995, 374:203-287
- ↑ Yankulov, Krassimir Y., and David L. Bentley. "Regulation of CDK7 substrate specificity by MAT1 and TFIIH." The EMBO Journal 16.7 (1997): 1638-1646.
- ↑ Wallenfang, Matthew R., and Geraldine Seydoux. "cdk-7 is required for mRNA transcription and cell cycle progression in Caenorhabditis elegans embryos." Proceedings of the National Academy of Sciences 99.8 (2002): 5527-5532.
- ↑ Ebmeier, Christopher C., et al. "Human TFIIH kinase CDK7 regulates transcription-associated chromatin modifications." Cell reports 20.5 (2017): 1173-1186.
- ↑ Kim YK et al., Recruitment of TFIIH to the HIV LTR is a rate-limiting step in the emergence of HIV from latency. EMBO J. 2006 Aug 9;25(15):3596-604
- ↑ Blau J , Xiao H , McCracken S , O'Hare P , Greenblatt J , Bentley D (1996) Three functional classes of transcriptional activation domains. Mol Cell Biol 16: 2044–2055
- ↑ Spilianakis C , Kretsovali A , Agalioti T , Makatounakis T , Thanos D , Papamatheakis J (2003) CIITA regulates transcription onset via Ser5-phosphorylation of RNA Pol II. EMBO J 22: 5125–5136
- ↑ Nissen RM , Yamamoto KR (2000) The glucocorticoid receptor inhibits NF-κB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev 14: 2314–2329
- ↑ Patel, Hetal, et al. "Expression of CDK7, cyclin H, and MAT1 is elevated in breast Cancer and is prognostic in estrogen receptor–Positive breast Cancer." Clinical Cancer Research 22.23 (2016): 5929-5938.
- ↑ Sun, Bowen, et al. "Inhibition of the transcriptional kinase CDK7 overcomes therapeutic resistance in HER2-positive breast cancers." Oncogene (2019): 1-14.
- ↑ Schneider, Eberhard, Mathias Montenarh, and Peter Wagner. "Regulation of CAK kinase activity by p53." Oncogene 17.21 (1998): 2733.
- ↑ Kwiatkowski, Nicholas, et al. "Targeting transcription regulation in cancer with a covalent CDK7 inhibitor." Nature 511.7511 (2014): 616.