Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases.

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifically, a neurotoxin or neurotoxicant– alters the normal activity of the nervous system in such a way as to cause permanent or reversible damage to nervous tissue. This can eventually disrupt or even kill neurons, which are cells that transmit and process signals in the brain and other parts of the nervous system. Neurotoxicity can result from organ transplants, radiation treatment, certain drug therapies, recreational drug use, exposure to heavy metals, bites from certain species of venomous snakes, pesticides, certain industrial cleaning solvents, fuels and certain naturally occurring substances. Symptoms may appear immediately after exposure or be delayed. They may include limb weakness or numbness, loss of memory, vision, and/or intellect, uncontrollable obsessive and/or compulsive behaviors, delusions, headache, cognitive and behavioral problems and sexual dysfunction. Chronic mold exposure in homes can lead to neurotoxicity which may not appear for months to years of exposure. All symptoms listed above are consistent with mold mycotoxin accumulation.

Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Zelapar, and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder. It has also been studied and used off-label for a variety of other indications, but has not been formally approved for any other use. The medication, in the form licensed for depression, has modest effectiveness for this condition that is similar to that of other antidepressants. Selegiline is provided as a swallowed tablet or capsule or an orally disintegrating tablet (ODT) for Parkinson's disease and as a patch applied to skin for depression.

Rasagiline, sold under the brand name Azilect among others, is a medication which is used in the treatment of Parkinson's disease. It is used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases. The drug is taken by mouth.

Pargyline, sold under the brand name Eutonyl among others, is a monoamine oxidase inhibitor (MAOI) medication which has been used to treat hypertension but is no longer marketed. It has also been studied as an antidepressant, but was never licensed for use in the treatment of depression. The drug is taken by mouth.
PC12 is a cell line derived from a pheochromocytoma of the rat adrenal medulla, that have an embryonic origin from the neural crest that has a mixture of neuroblastic cells and eosinophilic cells.

Droxidopa, also known as L-threo-dihydroxyphenylserine (L-DOPS) and sold under the brand names Northera and Dops among others, is sympathomimetic medication which is used in the treatment of hypotension and for other indications. It is taken by mouth.
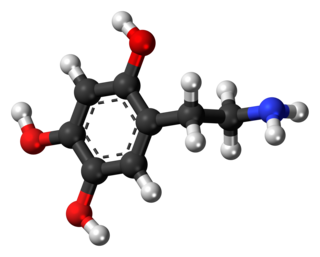
Oxidopamine, also known as 6-hydroxydopamine (6-OHDA) or 2,4,5-trihydroxyphenethylamine, is a synthetic monoaminergic neurotoxin used by researchers to selectively destroy dopaminergic and noradrenergic neurons in the brain.

Levoamphetamine is a stimulant medication which is used in the treatment of certain medical conditions. It was previously marketed by itself under the brand name Cydril, but is now available only in combination with dextroamphetamine in varying ratios under brand names like Adderall and Evekeo. The drug is known to increase wakefulness and concentration in association with decreased appetite and fatigue. Pharmaceuticals that contain levoamphetamine are currently indicated and prescribed for the treatment of attention deficit hyperactivity disorder (ADHD), obesity, and narcolepsy in some countries. Levoamphetamine is taken by mouth.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters.

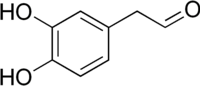
3,4-Dihydroxyphenylacetaldehyde (DOPAL), also known as dopamine aldehyde, is a metabolite of the monoamine neurotransmitter dopamine formed by monoamine oxidase (MAO).
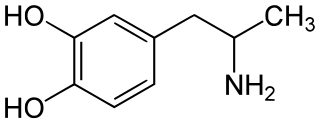
α-Methyldopamine (α-Me-DA), also known as 3,4-dihydroxyamphetamine or as catecholamphetamine, is a research chemical of the catecholamine and amphetamine families. It is a monoamine releasing agent and a metabolite of MDMA and MDA. The bis-glutathionyl metabolite of α-methyldopamine is slightly neurotoxic when directly injected into the brain's ventricles.

A disulfiram-like drug is a drug that causes an adverse reaction to alcohol leading to nausea, vomiting, flushing, dizziness, throbbing headache, chest and abdominal discomfort, and general hangover-like symptoms among others. These effects are caused by accumulation of acetaldehyde, a major but toxic metabolite of alcohol formed by the enzyme alcohol dehydrogenase. The reaction has been variously termed a disulfiram-like reaction, alcohol intolerance, and acetaldehyde syndrome.

Animal models of Parkinson's disease are essential in the research field and widely used to study Parkinson's disease. Parkinson's disease is a neurodegenerative disorder, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The loss of the dopamine neurons in the brain, results in motor dysfunction, ultimately causing the four cardinal symptoms of PD: tremor, rigidity, postural instability, and bradykinesia. It is the second most prevalent neurodegenerative disease, following Alzheimer's disease. It is estimated that nearly one million people could be living with PD in the United States.

The pharmacology of selegiline pertains to the pharmacodynamic and pharmacokinetic properties of the antiparkinsonian and antidepressant selegiline (L-deprenyl). Selegiline is available in a few different forms, including oral tablets and capsules, orally disintegrating tablets (ODTs), and transdermal patches. These forms have differing pharmacological properties.

A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine.

5-Hydroxyindoleacetaldehyde (5-HIAL), also known as 5-hydroxytryptaldehyde or as serotonin aldehyde, is an inactive metabolite and metabolic intermediate of the monoamine neurotransmitter serotonin.

3,4-Dihydroxyphenylglycolaldehyde (DOPEGAL), also known as 3,4-dihydroxymandelaldehyde (DHMAL) as well as norepinephrine aldehyde or epinephrine aldehyde, is a metabolite of the monoamine neurotransmitters norepinephrine and epinephrine. DOPEGAL is a noradrenergic neurotoxin.