
Yersinia pestis is a gram-negative, non-motile, coccobacillus bacterium without spores that is related to both Yersinia enterocolitica and Yersinia pseudotuberculosis, the pathogen from which Y. pestis evolved and responsible for the Far East scarlet-like fever. It is a facultative anaerobic organism that can infect humans via the Oriental rat flea. It causes the disease plague, which caused the Plague of Justinian and the Black Death, the deadliest pandemic in recorded history. Plague takes three main forms: pneumonic, septicemic, and bubonic. Yersinia pestis is a parasite of its host, the rat flea, which is also a parasite of rats, hence Y. pestis is a hyperparasite.

Q fever or query fever is a disease caused by infection with Coxiella burnetii, a bacterium that affects humans and other animals. This organism is uncommon, but may be found in cattle, sheep, goats, and other domestic mammals, including cats and dogs. The infection results from inhalation of a spore-like small-cell variant, and from contact with the milk, urine, feces, vaginal mucus, or semen of infected animals. Rarely, the disease is tick-borne. The incubation period can range from 9 to 40 days. Humans are vulnerable to Q fever, and infection can result from even a few organisms. The bacterium is an obligate intracellular pathogenic parasite.

Erysipelothrix rhusiopathiae is a Gram-positive, catalase-negative, rod-shaped, non-spore-forming, nonacid-fast, nonmotile bacterium. Distributed worldwide, E. rhusiopathiae is primarily considered an animal pathogen, causing the disease known as erysipelas that may affect a wide range of animals. Pigs, turkeys and laying hens are most commonly affected, but cases have been reported in other mammals, birds, fish, and reptiles. In pigs, the disease is known as diamond skin disease. The bacterium can also cause zoonotic infections in humans, called erysipeloid. The human disease called erysipelas is not caused by E. rhusiopathiae, but by various members of the genus Streptococcus.

Corynebacterium diphtheriae is the pathogenic bacterium that causes diphtheria. It is also known as the Klebs–Löffler bacillus, because it was discovered in 1884 by German bacteriologists Edwin Klebs (1834–1912) and Friedrich Löffler (1852–1915). The bacteria are usually harmless unless they are infected by a bacteriophage that carries a gene that gives rise to a toxin. This toxin causes the disease. Diphtheria is caused by the adhesion and infiltration of the bacteria into the mucosal layers of the body, primarily affecting the respiratory tract and the subsequent release of an endotoxin. The toxin has a localized effect on skin lesions, as well as a metastatic, proteolytic effects on other organ systems in severe infections. Originally a major cause of childhood mortality, diphtheria has been almost entirely eradicated due to the vigorous administration of the diphtheria vaccination in the 1910s.

Yersinia enterocolitica is a Gram-negative, rod-shaped bacterium, belonging to the family Yersiniaceae. It is motile at temperatures of 22–29°C (72–84°F), but becomes nonmotile at normal human body temperature. Y. enterocolitica infection causes the disease yersiniosis, which is an animal-borne disease occurring in humans, as well as in a wide array of animals such as cattle, deer, pigs, and birds. Many of these animals recover from the disease and become carriers; these are potential sources of contagion despite showing no signs of disease. The bacterium infects the host by sticking to its cells using trimeric autotransporter adhesins.

Yersinia pseudotuberculosis is a Gram-negative bacterium that causes Far East scarlet-like fever in humans, who occasionally get infected zoonotically, most often through the food-borne route. Animals are also infected by Y. pseudotuberculosis. The bacterium is urease positive.

Bovine malignant catarrhal fever (BMCF) is a fatal lymphoproliferative disease caused by a group of ruminant gamma herpes viruses including Alcelaphine gammaherpesvirus 1 (AlHV-1) and Ovine gammaherpesvirus 2 (OvHV-2) These viruses cause unapparent infection in their reservoir hosts, but are usually fatal in cattle and other ungulates such as deer, antelope, and buffalo. In Southern Africa the disease is known as snotsiekte, from the Afrikaans.
Fusobacterium necrophorum is a species of bacteria responsible for Lemierre's syndrome. It has also been known to cause sinusitis, mastoiditis, and odontogenic infections.
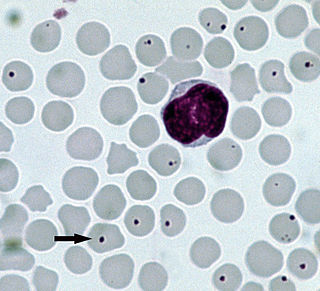
Anaplasmosis is a tick-borne disease affecting ruminants, dogs, and horses, and is caused by Anaplasma bacteria. Anaplasmosis is an infectious but not contagious disease. Anaplasmosis can be transmitted through mechanical and biological vector processes. Anaplasmosis can also be referred to as "yellow bag" or "yellow fever" because the infected animal can develop a jaundiced look. Other signs of infection include weight loss, diarrhea, paleness of the skin, aggressive behavior, and high fever.
Dichelobacter nodosus, formerly Bacteroides nodosus, is a Gram-negative, obligate anaerobe of the family Cardiobacteriaceae. It has polar fimbriae and is the causative agent of ovine foot rot as well as interdigital dermatitis. It is the lone species in the genus Dichelobacter.
Mycoplasma ovipneumoniae is a species of Mycoplasma bacteria that most commonly inhabits and affects ovine animals, first described in 1972. M. ovipneumoniae contributes to harmful pneumonia in sheep and goats. The duration and severity of M. ovipneumoniae varies from region to region.

Rhodococcus equi is a Gram-positive coccobacillus bacterium. The organism is commonly found in dry and dusty soil and can be important for diseases of domesticated animals. The frequency of infection can reach near 60%. R. equi is an important pathogen causing pneumonia in foals. Since 2008, R. equi has been known to infect wild boar and domestic pigs. R. equi can infect humans. At-risk groups are immunocompromised people, such as HIV-AIDS patients or transplant recipients. Rhodococcus infection in these patients resemble clinical and pathological signs of pulmonary tuberculosis. It is facultative intracellular.
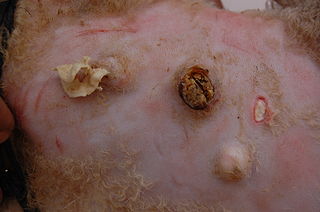
Caseous lymphadenitis (CLA) is an infectious disease caused by the bacterium Corynebacterium pseudotuberculosis, that affects the lymphatic system, resulting in abscesses in the lymph nodes and internal organs. It is found mostly in goats and sheep and at the moment it has no cure.
Edwardsiella tarda is a member of the family Hafniaceae. The bacterium is a facultatively anaerobic, small, motile, gram negative, straight rod with flagella. Infection causes Edwardsiella septicemia in channel catfish, eels, and flounder. Edwardsiella tarda is also found in largemouth bass and freshwater species such as rainbow trout. It is a zoonosis and can infect a variety of animals including fish, amphibians, reptiles, and mammals. Edwardsiella tarda has also been the cause of periodic infections for various animals within zoos. E. tarda has a worldwide distribution and can be found in pond water, mud, and the intestine of fish and other marine animals. It is spread by carrier animal feces.
Pigeon fever is a disease of horses, also known as dryland distemper or equine distemper, caused by the Gram-positive bacteria Corynebacterium pseudotuberculosis biovar equi. Infected horses commonly have swelling in the chest area, making it look similar to a "pigeon chest". This disease is common in dry areas. Pigeon fever is sometimes confused with strangles, another infection that causes abscesses.
Actinobacillus pleuropneumoniae, is a Gram-negative, facultative anaerobic, respiratory pathogen found in pigs. It was first reported in 1957, and was formally declared to be the causative agent of porcine pleuropneumonia in 1964. It was reclassified in 1983 after DNA studies showed it was more closely related to A. lignieresii.
Capripoxvirus is a genus of viruses in the subfamily Chordopoxvirinae and the family Poxviridae. Capripoxviruses are among the most serious of all animal poxviruses. All CaPV are notifiable diseases to the OIE. Sheep, goat, and cattle serve as natural hosts. These viruses cause negative economic consequences by damaging hides and wool and forcing the establishment of trade restrictions in response to an outbreak. The genus consists of three species: sheeppox virus (SPPV), goatpox virus (GTPV), and lumpy skin disease virus (LSDV). They share no serological relationship with camel pox, horse pox, or avian poxes. Capripoxviruses for sheeppox and goatpox infect only sheep and goat respectively. However, it is probable that North American relatives, the mountain goat and mountain sheep, may be susceptible to the strains but has not been experimentally proven. Lumpy skin disease virus affects primarily cattle, but studies have been shown that giraffes and impala are also susceptible to LSDV. Humans cannot be infected by Capripoxviruses.
Bordetella avium is a gram negative, nonfermentative, strictly aerobic, motile bacterium from the genus Bordetella which has been isolated from patients with respiratory disease. B. avium has a global distribution, that mainly affects young domesticated turkeys. The disease in birds is called bordetellosis, and is largely associated with confined spaces and multi-aged flocks where management practices are sub optimal. In most infections, mortality is typically low but morbidity is very high.

Actinomyces bovis is a branching, Gram-positive, rod-shaped bacterium of the genus Actinomyces. It is the causative agent of lumpy jaw in cattle, and occasionally causes actinomycosis infections in humans. A. bovis normally populates the gastrointestinal tract of healthy ruminants, but is opportunistic in nature and will move into tissues through ulcerations or abrasions of the mucosa to cause infection. The disease occurs when there is physical damage to the tissue of the mouth, allowing the bacteria to colonize the deep tissue and bone, typically affecting the mandible and maxilla. Actinomycosis is pathognomonic for abscesses containing "sulfur" granules, and its colonies appear basophilic with club-shaped reaction products on a histological preparation. Lumpy jaw is commonly treated with broad-spectrum antibiotics with varying success, and can be a major economic loss for producers in countries where it is endemic. Because this organism is zoonotic, it is a human health concern and can cause granulomas, abscesses, skin lesions, and bronchopneumonia.
Histophilus somni is a non-motile, gram-negative, rod or coccobacillus shaped, facultative anaerobe bacterial species belonging to the family Pasteurellaceae. Prior to 2003, it was thought Haemophilus somnus, Histophilus ovis, and Histophilus agni were three different species, but now are all classified as Histophilus somni. Histophilus somni is a commensal bacteria of mucous membranes of the upper respiratory tract and reproductive tract with a global prevalence and is found in cattle and other small ruminants. Histophilus somni is also a known causative agent that is a part of the Bovine Respiratory Disease (BRD) complex, which typically involves multiple pathogens residing together in biofilm environments. Histophilus somni may also cause Histophilosus symptoms and clinical presentation will depend on the tissue affected. When disease does occur, it can be difficult to catch in time and is often diagnosed on post mortem. This means that treatment often involves metaphylactic mass treatment or no treatment at all. This organism is more fastidious than others and requires knowledge for sample collection, storage and culture. Genomic studies related to this bacteria have enabled scientist to pin point antibiotic resistance genes.












