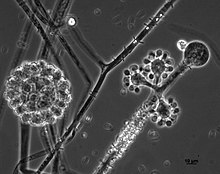
Zygomycota, or zygote fungi, is a former division or phylum of the kingdom Fungi. The members are now part of two phyla: the Mucoromycota and Zoopagomycota. Approximately 1060 species are known. They are mostly terrestrial in habitat, living in soil or on decaying plant or animal material. Some are parasites of plants, insects, and small animals, while others form symbiotic relationships with plants. Zygomycete hyphae may be coenocytic, forming septa only where gametes are formed or to wall off dead hyphae. Zygomycota is no longer recognised as it was not believed to be truly monophyletic.
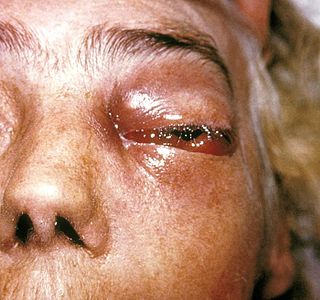
Zygomycosis is the broadest term to refer to infections caused by bread mold fungi of the zygomycota phylum. However, because zygomycota has been identified as polyphyletic, and is not included in modern fungal classification systems, the diseases that zygomycosis can refer to are better called by their specific names: mucormycosis, phycomycosis and basidiobolomycosis. These rare yet serious and potentially life-threatening fungal infections usually affect the face or oropharyngeal cavity. Zygomycosis type infections are most often caused by common fungi found in soil and decaying vegetation. While most individuals are exposed to the fungi on a regular basis, those with immune disorders (immunocompromised) are more prone to fungal infection. These types of infections are also common after natural disasters, such as tornadoes or earthquakes, where people have open wounds that have become filled with soil or vegetative matter.

The Mucorales is the largest and best-studied order of zygomycete fungi. Members of this order are sometimes called pin molds. The term mucormycosis is now preferred for infections caused by molds belonging to the order Mucorales.

Mortierella species are soil fungi belonging to the order Mortierellales within the subphylum Mortierellomycotina. The widespread genus contains about 85 species.

Apophysomyces is a genus of filamentous fungi that are commonly found in soil and decaying vegetation. Species normally grow in tropical to subtropical regions.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.

Mucor mucedo, commonly known as the common pinmould, is a fungal plant pathogen and member of the phylum Mucoromycota and the genus Mucor. Commonly found on soil, dung, water, plants and moist foods, Mucor mucedo is a saprotrophic fungus found world-wide with 85 known strains. It is often mistaken for Rhizopus rots on fruits due to similar mould growth shape and colour. Contrastingly, however, Mucor mucedo is found to grow on a wide range of stored grains and plants, including cucumber and tomato. Discovered in Italy in 1729 by P.A. Micheli and later noted by Carl Linnaeus in 1753 in the Species Plantarum, Mucor mucedo was originally classified as Mucor vulgaris by Micheli but later classified synonymous under name Mucor mucedo. The species was redescribed as Ascophora mucedo by H.J. Tode in 1790 but this type resided in a stoloniferous habitat and was later made the type of new genus Rhizopus.

Mucor racemosus is a rapidly growing, weedy mould belonging to the division Mucoromycota. It is one of the earliest fungi to be grown in pure culture and was first isolated in 1886. It has a worldwide distribution and colonizes many habitats such as vegetational products, soil and houses. The fungus is mostly known for its ability to exhibit both filamentous and yeast-like morphologies, often referred to as dimorphism. Stark differences are seen in both forms and conditions of the environment heavily affect the phases of the M. racemosus. Like many fungi, it also reproduces both sexually and asexually. The dimorphic capacity of this species has been proposed as an important factor in its pathogenicity and has enhanced the industrial importance. This species is considered an opportunistic pathogen, generally limited to immunocompromised individuals. It also been associated with allergy and inflammations of facial sinuses. Its association with allergy has made it a common fungus used in allergen medical testing. Industrial use of the fungus is in the production of enzymes and the manufacture of certain dairy foods.
Pathogenic fungi are fungi that cause disease in humans or other organisms. Although fungi are eukaryotic, many pathogenic fungi are microorganisms. Approximately 300 fungi are known to be pathogenic to humans; their study is called "medical mycology". Fungal infections are estimated to kill more people than either tuberculosis or malaria—about two million people per year.

Mucormycosis, also known as black fungus, is a serious fungal infection that comes under fulminant fungal sinusitis, usually in people who are immunocompromised. It is curable only when diagnosed early. Symptoms depend on where in the body the infection occurs. It most commonly infects the nose, sinuses, eyes and brain resulting in a runny nose, one-sided facial swelling and pain, headache, fever, blurred vision, bulging or displacement of the eye (proptosis), and tissue death. Other forms of disease may infect the lungs, stomach and intestines, and skin.
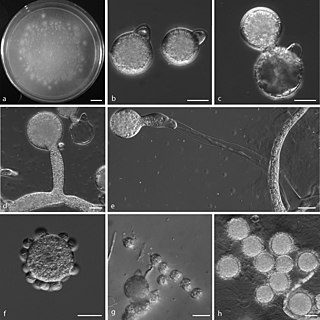
Conidiobolus coronatus is a saprotrophic fungus, first described by Costantin in 1897 as Boudierella coronata. Though this fungus has also been known by the name Entomophthora coronata, the correct name is Conidiobolus coronatus. C. coronatus is able to infect humans and animals, and the first human infection with C. coronatus was reported in Jamaica in 1965.
Cunninghamella elegans is a species of fungus in the genus Cunninghamella found in soil.

Apophysomyces variabilis is an emerging fungal pathogen that can cause serious and sometimes fatal infection in humans. This fungus is a soil-dwelling saprobe with tropical to subtropical distribution. It is a zygomycete that causes mucormycosis, an infection in humans brought about by fungi in the order Mucorales. Infectious cases have been reported globally in locations including the Americas, Southeast Asia, India, and Australia. Apophysomyces variabilis infections are not transmissible from person to person.

Lichtheimia corymbifera is a thermophilic fungus in the phylum Zygomycota. It normally lives as a saprotrophic mold, but can also be an opportunistic pathogen known to cause pulmonary, CNS, rhinocerebral, or cutaneous infections in animals and humans with impaired immunity.
Cunninghamella bertholletiae is a species of zygomycetous fungi in the order Mucorales. It is found globally, with increased prevalence in Mediterranean and subtropical climates. It typically grows as a saprotroph and is found in a wide variety of substrates, including soil, fruits, vegetables, nuts, crops, and human and animal waste. Although infections are still rare, C. betholletiae is emerging as an opportunistic human pathogen, predominantly in immunocompromised people, leukemia patients, and people with uncontrolled diabetes. Cunninghamella bertholletiae infections are often highly invasive, and can be more difficult to treat with antifungal drugs than infections with other species of the Mucorales, making prompt and accurate recognition and diagnosis of mycoses caused by this fungus an important medical concern.

Rhizopus stolonifer is commonly known as black bread mold. It is a member of Zygomycota and considered the most important species in the genus Rhizopus. It is one of the most common fungi in the world and has a global distribution although it is most commonly found in tropical and subtropical regions. It is a common agent of decomposition of stored foods. Like other members of the genus Rhizopus, R. stolonifer grows rapidly, mostly in indoor environments.

Mucor circinelloides is a dimorphic fungus belonging to the Order Mucorales. It has a worldwide distribution, found mostly in soil, dung and root vegetables. This species is described as not known to be able to produce mycotoxins, however it has been frequently reported to infect animals such as cattle and swine, as well as fowl, platypus and occasionally humans. Ketoacidotic patients are particularly at risk for infection by M. circinelloides.
Mycotypha microspora, also known as Microtypha microspora, is a filamentous fungus in the division Zygomycota. It was discovered in a Citrus aurantium peel in 1932 by E. Aline Fenner, who proposed a new genus Mycotypha to accommodate it. Mycotypha africana, which is another species in the genus Mycotypha, is closely related to M. microspora. The fungus has subsequently been isolated from both outdoor and indoor settings around the world, and is typically found in soil and dung. The species rarely causes infections in humans, but has recently been involved in the clinical manifestation of the life-threatening disease mucormycosis.

Mucoromycota is a division within the kingdom fungi. It includes a diverse group of various molds, including the common bread molds Mucor and Rhizopus. It is a sister phylum to Dikarya.
Calcarisporiellaceae is a family of fungi within the subkingdom Mucoromycota. It is the only family in the order Calcarisporiellales, class Calcarisporiellomycetes, subphylum Calcarisporiellomycotina and phylum Calcarisporiellomycota. It contains two known genera, Calcarisporiella and Echinochlamydosporium. The two genera each have one species.