
Cenani–Lenz syndactylism, also known as Cenani–Lenz syndrome or Cenani–syndactylism, is an autosomal recessive congenital malformation syndrome involving both upper and lower extremities.

Aniridia is the absence of the iris, a muscular structure that opens and closes the pupil to allow light into the eye. It is also responsible for eye color. Without it, the central eye appears all black. It can be congenital, in which both eyes are usually involved, or caused by a penetrant injury. Isolated aniridia is a congenital disorder that is not limited to a defect in iris development, but is a panocular condition with macular and optic nerve hypoplasia, cataract, and corneal changes. Vision may be severely compromised and the disorder is frequently associated with some ocular complications: nystagmus, amblyopia, buphthalmos, and cataract. Aniridia in some individuals occurs as part of a syndrome, such as WAGR syndrome, or Gillespie syndrome.
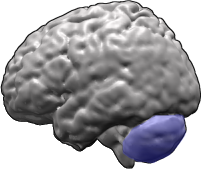
Spinocerebellar ataxia (SCA) is a progressive, degenerative, genetic disease with multiple types, each of which could be considered a neurological condition in its own right. An estimated 150,000 people in the United States have a diagnosis of spinocerebellar ataxia at any given time. SCA is hereditary, progressive, degenerative, and often fatal. There is no known effective treatment or cure. SCA can affect anyone of any age. The disease is caused by either a recessive or dominant gene. In many cases people are not aware that they carry a relevant gene until they have children who begin to show signs of having the disorder. Currently, research is being conducted at Universities, such as the University of Minnesota, to elucidate many of the unknown characteristics of the disease.

Behr syndrome is characterized by the association of early-onset optic atrophy with spinocerebellar degeneration resulting in ataxia, pyramidal signs, peripheral neuropathy and developmental delay.
Cerebellar ataxia is a form of ataxia originating in the cerebellum. Non-progressive congenital ataxia (NPCA) is a classical presentation of cerebral ataxias.

Paired box protein Pax-6, also known as aniridia type II protein (AN2) or oculorhombin, is a protein that in humans is encoded by the PAX6 gene.

Macular hypoplasia is a rare medical condition involving the underdevelopment of the macula, a small area on the retina responsible for seeing in detail and sensing light. Macular hypoplasia is often associated with albinism.

Purine nucleoside phosphorylase deficiency is a rare autosomal recessive metabolic disorder which results in immunodeficiency.
Vici syndrome, also called immunodeficiency with cleft lip/palate, cataract, hypopigmentation and absent corpus callosum, is a rare autosomal recessive congenital disorder characterized by albinism, agenesis of the corpus callosum, cataracts, cardiomyopathy, severe psychomotor retardation, seizures, immunodeficiency and recurrent severe infections. To date, about 50 cases have been reported.
Uner Tan syndrome (UTS) is a syndrome that was discovered by the Turkish evolutionary biologist Üner Tan. People affected by UTS walk with a quadrupedal locomotion and often have severe learning disabilities. Tan postulated that this is an example of "reverse evolution" (atavism). The proposed syndrome was featured in the 2006 BBC2 documentary The Family That Walks On All Fours.

Norman–Roberts syndrome is a rare form of microlissencephaly caused by a mutation in the RELN gene. A small number of cases have been described. The syndrome was first reported by Margaret Grace Norman and M. Roberts et al. in 1976.
VLDLR-associated cerebellar hypoplasia (VLDLRCH) is a rare autosomal recessive condition caused by a disruption of the VLDLR gene. First described as a form of cerebral palsy in the 1970s, it is associated with parental consanguinity and is found in secluded communities, with a number of cases described in Hutterite families.

Woodhouse–Sakati syndrome, is a rare autosomal recessive multisystem disorder which causes malformations throughout the body, and deficiencies affecting the endocrine system.

Pontocerebellar hypoplasia (PCH) is a heterogeneous group of rare neurodegenerative disorders caused by genetic mutations and characterised by progressive atrophy of various parts of the brain such as the cerebellum or brainstem. Where known, these disorders are inherited in an autosomal recessive fashion. There is no known cure for PCH.

Autosomal recessive cerebellar ataxia type 1 (ARCA1) is a condition characterized by progressive problems with movement. Signs and symptoms of the disorder first appear in early to mid-adulthood. People with this condition initially experience impaired speech (dysarthria), problems with coordination and balance (ataxia), or both. They may also have difficulty with movements that involve judging distance or scale (dysmetria). Other features of ARCA1 include abnormal eye movements (nystagmus) and problems following the movements of objects with their eyes. The movement problems are slowly progressive, often resulting in the need for a cane, walker, or wheelchair.
Oculomotor apraxia (OMA) is the absence or defect of controlled, voluntary, and purposeful eye movement. It was first described in 1952 by the American ophthalmologist David Glendenning Cogan. People with this condition have difficulty moving their eyes horizontally and moving them quickly. The main difficulty is in saccade initiation, but there is also impaired cancellation of the vestibulo-ocular reflex. Patients have to turn their head in order to compensate for the lack of eye movement initiation in order to follow an object or see objects in their peripheral vision, but they often exceed their target. There is controversy regarding whether OMA should be considered an apraxia, since apraxia is the inability to perform a learned or skilled motor action to command, and saccade initiation is neither a learned nor a skilled action.

COACH syndrome, also known as Joubert syndrome with hepatic defect, is a rare autosomal recessive genetic disease. The name is an acronym of the defining signs: cerebellar vermis aplasia, oligophrenia, congenital ataxia, coloboma and hepatic fibrosis. The condition is associated with moderate intellectual disability. It falls under the category of a Joubart Syndrome-related disorder (JSRD).
Autosomal dominant cerebellar ataxia, deafness, and narcolepsy (ADCADN) is a rare progressive genetic disorder that primarily affects the nervous system and is characterized by sensorineural hearing loss, narcolepsy with cataplexy, and dementia later in life. People with this disorder usually start showing symptoms when they are in their early-mid adulthoods. It is a type of autosomal dominant cerebellar ataxia.









