
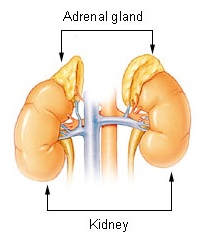
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.

The adrenal cortex is the outer region and also the largest part of the adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.

Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.

Primary aldosteronism (PA), also known as primary hyperaldosteronism, refers to the excess production of the hormone aldosterone from the adrenal glands, resulting in low renin levels and high blood pressure. This abnormality is a paraneoplastic syndrome. About 35% of the cases are caused by a single aldosterone-secreting adenoma, a condition known as Conn's syndrome.

Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances. The primary mineralocorticoid is aldosterone.
Adrenal insufficiency is a condition in which the adrenal glands do not produce adequate amounts of steroid hormones. The adrenal glands—also referred to as the adrenal cortex—normally secrete glucocorticoids, mineralocorticoids, and androgens. These hormones are important in regulating blood pressure, electrolytes, and metabolism as a whole. Deficiency of these hormones leads to symptoms ranging from abdominal pain, vomiting, muscle weakness and fatigue, low blood pressure, depression, mood and personality changes to organ failure and shock. Adrenal crisis may occur if a person having adrenal insufficiency experiences stresses, such as an accident, injury, surgery, or severe infection; this is a life-threatening medical condition resulting from severe deficiency of cortisol in the body. Death may quickly follow.
Congenital adrenal hyperplasia due to 17α-hydroxylase deficiency is an uncommon form of congenital adrenal hyperplasia (CAH) resulting from a mutation in the gene CYP17A1, which produces the enzyme 17α-hydroxylase. It causes decreased synthesis of cortisol and sex hormones, with resulting increase in mineralocorticoid production. Thus, common symptoms include mild cortisol deficiency, ambiguous genitalia in men or amenorrhea at puberty in women, and hypokalemic hypertension. However, partial (incomplete) deficiency often has inconsistent symptoms between patients, and affected women may be asymptomatic except for infertility.

Hypoaldosteronism is an endocrinological disorder characterized by decreased levels of the hormone aldosterone. Similarly, isolated hypoaldosteronism is the condition of having lowered aldosterone without corresponding changes in cortisol.

The zona glomerulosa of the adrenal gland is the most superficial layer of the adrenal cortex, lying directly beneath the renal capsule. Its cells are ovoid and arranged in clusters or arches.
Secondary hypertension is a type of hypertension which has a specific and identifiable underlying primary cause. It is much less common than essential hypertension, affecting only 5-10% of hypertensive patients. It has many different causes including obstructive sleep apnea, kidney disease, endocrine diseases, and tumors. The cause of secondary hypertension varies significantly with age. It also can be a side effect of many medications.

Apparent mineralocorticoid excess is an autosomal recessive disorder causing hypertension, hypernatremia and hypokalemia. It results from mutations in the HSD11B2 gene, which encodes the kidney isozyme of 11β-hydroxysteroid dehydrogenase type 2. In an unaffected individual, this isozyme inactivates circulating cortisol to the less active metabolite cortisone. The inactivating mutation leads to elevated local concentrations of cortisol in the aldosterone sensitive tissues like the kidney. Cortisol at high concentrations can cross-react and activate the mineralocorticoid receptor due to the non-selectivity of the receptor, leading to aldosterone-like effects in the kidney. This is what causes the hypokalemia, hypertension, and hypernatremia associated with the syndrome. Patients often present with severe hypertension and end-organ changes associated with it like left ventricular hypertrophy, retinal, renal and neurological vascular changes along with growth retardation and failure to thrive. In serum both aldosterone and renin levels are low.
In humans and other animals, the adrenocortical hormones are hormones produced by the adrenal cortex, the outer region of the adrenal gland. These polycyclic steroid hormones have a variety of roles that are crucial for the body's response to stress, and they also regulate other functions in the body. Threats to homeostasis, such as injury, chemical imbalances, infection, or psychological stress, can initiate a stress response. Examples of adrenocortical hormones that are involved in the stress response are aldosterone and cortisol. These hormones also function in regulating the conservation of water by the kidneys and glucose metabolism, respectively.
Pseudohyperaldosteronism is a medical condition which mimics the effects of elevated aldosterone (hyperaldosteronism) by presenting with high blood pressure, low blood potassium levels (hypokalemia), metabolic alkalosis, and low levels of plasma renin activity (PRA). However, unlike hyperaldosteronism, this conditions exhibits low or normal levels of aldosterone in the blood. Causes include genetic disorders, acquired conditions, metabolic disorders, and dietary imbalances including excessive consumption of licorice. Confirmatory diagnosis depends on the specific cause and may involve blood tests, urine tests, or genetic testing; however, all forms of this condition exhibit abnormally low concentrations of both plasma renin activity (PRA) and plasma aldosterone concentration (PAC) which differentiates this group of conditions from other forms of secondary hypertension. Treatment is tailored to the specific cause and focuses on symptom control, blood pressure management, and avoidance of triggers.

11-Deoxycorticosterone (DOC), or simply deoxycorticosterone, also known as 21-hydroxyprogesterone, as well as desoxycortone (INN), deoxycortone, and cortexone, is a steroid hormone produced by the adrenal gland that possesses mineralocorticoid activity and acts as a precursor to aldosterone. It is an active (Na+-retaining) mineralocorticoid. As its names indicate, 11-deoxycorticosterone can be understood as the 21-hydroxy-variant of progesterone or as the 11-deoxy-variant of corticosterone.

Aldosterone synthase, also called steroid 18-hydroxylase, corticosterone 18-monooxygenase or P450C18, is a steroid hydroxylase cytochrome P450 enzyme involved in the biosynthesis of the mineralocorticoid aldosterone and other steroids. The enzyme catalyzes sequential hydroxylations of the steroid angular methyl group at C18 after initial 11β-hydroxylation. It is encoded by the CYP11B2 gene in humans.

Steroid 11β-hydroxylase, also known as steroid 11β-monooxygenase, is a steroid hydroxylase found in the zona glomerulosa and zona fasciculata of the adrenal cortex. Named officially the cytochrome P450 11B1, mitochondrial, it is a protein that in humans is encoded by the CYP11B1 gene. The enzyme is involved in the biosynthesis of adrenal corticosteroids by catalyzing the addition of hydroxyl groups during oxidation reactions.
The ACTH test is a medical test usually requested and interpreted by endocrinologists to assess the functioning of the adrenal glands' stress response by measuring the adrenal response to adrenocorticotropic hormone or another corticotropic agent such as tetracosactide or alsactide (Synchrodyn). ACTH is a hormone produced in the anterior pituitary gland that stimulates the adrenal glands to release cortisol, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and aldosterone.
Familial hyperaldosteronism is a group of inherited conditions in which the adrenal glands, which are small glands located on top of each kidney, produce too much of the hormone aldosterone. Excess aldosterone causes the kidneys to retain more salt than normal, which in turn increases the body's fluid levels and causes high blood pressure. People with familial hyperaldosteronism may develop severe high blood pressure, often early in life. Without treatment, hypertension increases the risk of strokes, heart attacks, and kidney failure. There are other forms of hyperaldosteronism that are not inherited.
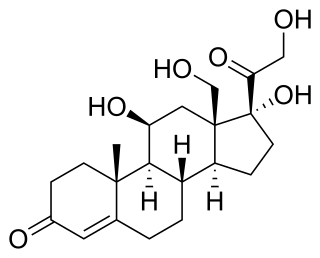
18-Hydroxycortisol is an endogenous steroid, a metabolite of cortisol.

18-Oxocortisol is an endogenous steroid, a metabolite of cortisol.










