GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved G domain common to many GTPases.
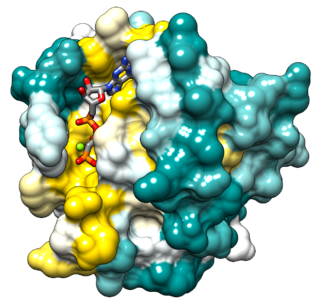
Ras is a family of related proteins which is expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells. Ras is the prototypical member of the Ras superfamily of proteins, which are all related in 3D structure and regulate diverse cell behaviours.
Small GTPases, also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases.
The Rab family of proteins is a member of the Ras superfamily of small G proteins. Approximately 70 types of Rabs have now been identified in humans. Rab proteins generally possess a GTPase fold, which consists of a six-stranded beta sheet which is flanked by five alpha helixes. Rab GTPases regulate many steps of membrane trafficking, including vesicle formation, vesicle movement along actin and tubulin networks, and membrane fusion. These processes make up the route through which cell surface proteins are trafficked from the Golgi to the plasma membrane and are recycled. Surface protein recycling returns proteins to the surface whose function involves carrying another protein or substance inside the cell, such as the transferrin receptor, or serves as a means of regulating the number of a certain type of protein molecules on the surface.

Prenylation is the addition of hydrophobic molecules to a protein or chemical compound. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups have been shown to be important for protein–protein binding through specialized prenyl-binding domains.

Rab geranylgeranyltransferase also known as (protein) geranylgeranyltransferase II is one of the three prenyltransferases. It transfers (usually) two geranylgeranyl groups to the cystein(s) at the C-terminus of Rab proteins.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.

GTP-binding protein regulators regulate G proteins in several different ways. Small GTPases act as molecular switches in signaling pathways, which act to regulate functions of other proteins. They are active or 'ON' when it is bound to GTP and inactive or 'OFF' when bound to GDP. Activation and deactivation of small GTPases can be regarded as occurring in a cycle, between the GTP-bound and GDP-bound form, regulated by other regulatory proteins.

Rab escort protein 1 (REP1) also known as rab proteins geranylgeranyltransferase component A 1 is an enzyme that in humans is encoded by the CHM gene.
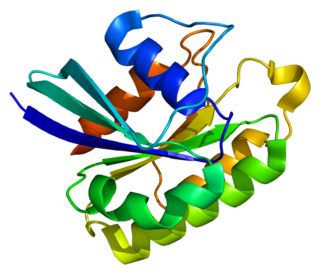
Ras-related protein Rab-5A is a protein that in humans is encoded by the RAB5A gene.

Rab GDP dissociation inhibitor alpha is a protein that in humans is encoded by the GDI1 gene.
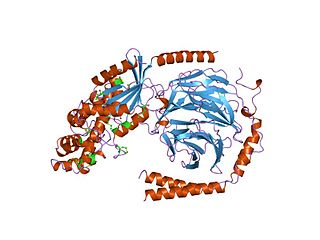
G alpha subunits are one of the three types of subunit of guanine nucleotide binding proteins, which are membrane-associated, heterotrimeric G proteins.

Rho GDP-dissociation inhibitor 1 is a protein that in humans is encoded by the ARHGDIA gene.

Rho GDP-dissociation inhibitor 2 is a protein that in humans is encoded by the ARHGDIB gene. Aliases of this gene include RhoGDI2, GDID4, Rho GDI 2, and others.

Rab GDP dissociation inhibitor beta is a protein that in humans is encoded by the GDI2 gene.

RABAC1 is a gene that in humans encodes the protein Prenylated Rab acceptor 1, also called PRA1, PRAF1, or RABAC1. It is highly conserved in eukaryotes. The protein is localized to Golgi and late endosomes, where it plays a role in vesicular trafficking, lipid transport and cell migration.

Rho GDP-dissociation inhibitor 3 is a protein that in humans is encoded by the ARHGDIG gene.

GoLoco motif is a protein structural motif.
The TBC (Tre-2/Bub2/Cdc16) is identified as a domain of some proteins or as a protein motif and widely recognized as a conserved one that includes approximately 200 amino acids in all eukaryotes.
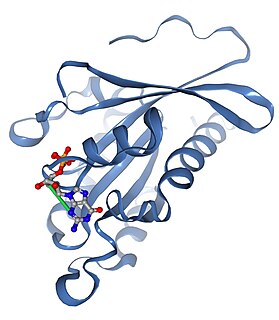
Ras-related protein Rab-2B is a protein that in humans is encoded by the RAB2B gene.
















