
Lipoxygenases (LOX) are a family of (non-heme) iron-containing enzymes, more specifically oxidative enzymes, most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4-pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.

Vernolic acid is a long chain fatty acid that is monounsaturated and contains an epoxide. It is a cis epoxide derived from the C12–C13 alkene of linoleic acid. Vernolic acid was first definitively characterized in 1954 and its absolute configuration determined in 1966. It is a major component in vernonia oil, which is produced in abundance by the genera Vernonia and Euphorbia and is a potentially useful biofeedstock.
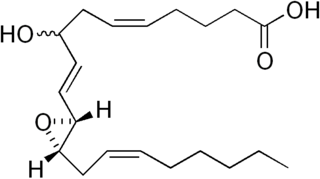
Hepoxilins (Hx) are a set of epoxyalcohol metabolites of polyunsaturated fatty acids (PUFA), i.e. they possess both an epoxide and an alcohol residue. HxA3, HxB3, and their non-enzymatically formed isomers are nonclassic eicosanoid derived from acid the (PUFA), arachidonic acid. A second group of less well studied hepoxilins, HxA4, HxB4, and their non-enzymatically formed isomers are nonclassical eicosanoids derived from the PUFA, eicosapentaenoic acid. Recently, 14,15-HxA3 and 14,15-HxB3 have been defined as arachidonic acid derivatives that are produced by a different metabolic pathway than HxA3, HxB3, HxA4, or HxB4 and differ from the aforementioned hepoxilins in the positions of their hydroxyl and epoxide residues. Finally, hepoxilin-like products of two other PUFAs, docosahexaenoic acid and linoleic acid, have been described. All of these epoxyalcohol metabolites are at least somewhat unstable and are readily enzymatically or non-enzymatically to their corresponding trihydroxy counterparts, the trioxilins (TrX). HxA3 and HxB3, in particular, are being rapidly metabolized to TrXA3, TrXB3, and TrXC3. Hepoxilins have various biological activities in animal models and/or cultured mammalian tissues and cells. The TrX metabolites of HxA3 and HxB3 have less or no activity in most of the systems studied but in some systems retain the activity of their precursor hepoxilins. Based on these studies, it has been proposed that the hepoxilins and trioxilins function in human physiology and pathology by, for example, promoting inflammation responses and dilating arteries to regulate regional blood flow and blood pressure.
In enzymology, a linoleate 11-lipoxygenase (EC 1.13.11.45) is an enzyme that catalyzes the chemical reaction
In enzymology, a linoleate diol synthase (EC 1.13.11.44) is an enzyme that catalyzes the chemical reaction

In enzymology, an allene-oxide cyclase is an enzyme that belongs to the family of isomerases, specifically a class of other intramolecular oxidoreductases. The systematic name of this enzyme class is (9Z)-(13S)-12,13-epoxyoctadeca-9,11,15-trienoate isomerase (cyclizing).
In enzymology, a polyenoic fatty acid isomerase is an enzyme that catalyzes the chemical reaction

In enzymology, a prostaglandin-D synthase is an enzyme that catalyzes the chemical reaction
The enzyme leukotriene-C4 synthase (EC 4.4.1.20) catalyzes the reaction
The enzyme prephenate dehydratase (EC 4.2.1.51) catalyzes the chemical reaction
Epoxygenases are a set of membrane-bound, heme-containing cytochrome P450 enzymes that metabolize polyunsaturated fatty acids (PUFAs) to epoxide products that have a range of biological activities.
Linoleate 8R-lipoxygenase (EC 1.13.11.60, linoleic acid 8R-dioxygenase, 5,8-LDS (bifunctional enzyme), 7,8-LDS (bifunctional enzyme), 5,8-linoleate diol synthase (bifunctional enzyme), 7,8-linoleate diol synthase (bifunctional enzyme), PpoA) is an enzyme with systematic name linoleate:oxygen (8R)-oxidoreductase. This enzyme catalyses the following chemical reaction
Linoleate 10R-lipoxygenase (EC 1.13.11.62, 10R-DOX, (10R)-dioxygenase, 10R-dioxygenase) is an enzyme with systematic name linoleate:oxygen (10R)-oxidoreductase. This enzyme catalyses the following chemical reaction
Colneleate synthase (EC 4.2.1.121, 9-divinyl ether synthase, 9-DES, CYP74D, CYP74D1, CYP74 cytochrome P-450, DES1) is an enzyme with systematic name (8E)-9-((1E,3E)-nona-1,3-dien-1-yloxy)non-8-enoate synthase. This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8R-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -5,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8S-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -7,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
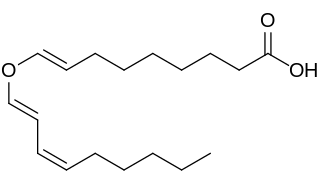
Divinylether fatty acids contain a fatty acid chemically combined with a doubly unsaturated carbon chain linked by an oxygen atom (ether).
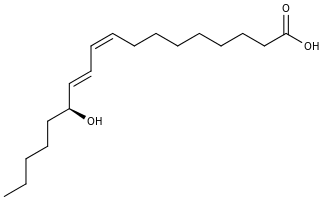
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.

Hydroperoxide lyases are enzymes that catalyze the cleavage of C-C bonds in the hydroperoxides of fatty acids. They belong to the cytochrome P450 enzyme family.










