Gibberellins (GAs) are plant hormones that regulate various developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence. GAs are one of the longest-known classes of plant hormone. It is thought that the selective breeding of crop strains that were deficient in GA synthesis was one of the key drivers of the "green revolution" in the 1960s, a revolution that is credited to have saved over a billion lives worldwide.

Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.

Indole-3-acetic acid is the most common naturally occurring plant hormone of the auxin class. It is the best known of the auxins, and has been the subject of extensive studies by plant physiologists. IAA is a derivative of indole, containing a carboxymethyl substituent. It is a colorless solid that is soluble in polar organic solvents.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Glucobrassicin is a type of glucosinolate that can be found in almost all cruciferous plants, such as cabbages, broccoli, mustards, and woad. As for other glucosinolates, degradation by the enzyme myrosinase is expected to produce an isothiocyanate, indol-3-ylmethylisothiocyanate. However, this specific isothiocyanate is expected to be highly unstable, and has indeed never been detected. The observed hydrolysis products when isolated glucobrassicin is degraded by myrosinase are indole-3-carbinol and thiocyanate ion, which are envisioned to result from a rapid reaction of the unstable isothiocyanate with water. However, a large number of other reaction products are known, and indole-3-carbinol is not the dominant degradation product when glucosinolate degradation takes place in crushed plant tissue or in intact plants.
Rhodococcus fascians is a Gram positive bacterial phytopathogen that causes leafy gall disease. R. fascians is the only phytopathogenic member of the genus Rhodococcus; its host range includes both dicotyledonous and monocotyledonous hosts. Because it commonly afflicts tobacco (Nicotiana) plants, it is an agriculturally significant pathogen.

Squalene monooxygenase is a eukaryotic enzyme that uses NADPH and diatomic oxygen to oxidize squalene to 2,3-oxidosqualene. Squalene epoxidase catalyzes the first oxygenation step in sterol biosynthesis and is thought to be one of the rate-limiting enzymes in this pathway. In humans, squalene epoxidase is encoded by the SQLE gene. Several eukaryote genomes lack a squalene monooxygenase encoding gene, but instead encode an alternative squalene epoxidase that performs the same task.
In enzymology, a dihydrokaempferol 4-reductase (EC 1.1.1.219) is an enzyme that catalyzes the chemical reaction

Malate dehydrogenase (oxaloacetate-decarboxylating) (NADP+) (EC 1.1.1.40) or NADP-malic enzyme (NADP-ME) is an enzyme that catalyzes the chemical reaction in the presence of a bivalent metal ion:
In enzymology, an indole-3-acetaldehyde oxidase (EC 1.2.3.7) is an enzyme that catalyzes the chemical reaction
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:
The enzyme indole-3-glycerol-phosphate lyase catalyzes the chemical reaction
The enzyme indolepyruvate decarboxylase (EC 4.1.1.74) catalyzes the chemical reaction
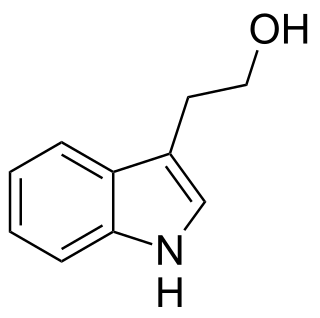
Tryptophol is an aromatic alcohol that induces sleep in humans. It is found in wine as a secondary product of ethanol fermentation. It was first described by Felix Ehrlich in 1912. It is also produced by the trypanosomal parasite in sleeping sickness.
Tryptophan N-monooxygenase (EC 1.14.13.125, tryptophan N-hydroxylase, CYP79B1, CYP79B2, CYP79B3) is an enzyme with systematic name L-tryptophan,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Indole-2-monooxygenase (EC 1.14.13.137, BX2 (gene), CYP71C4 (gene)) is an enzyme with systematic name indole,NAD(P)H:oxygen oxidoreductase (2-hydroxylating). This enzyme catalyses the following chemical reaction
(2,2,3-Trimethyl-5-oxocyclopent-3-enyl)acetyl-CoA 1,5-monooxygenase (EC 1.14.13.160, 2-oxo-Delta3-4,5,5-trimethylcyclopentenylacetyl-CoA monooxygenase, 2-oxo-Delta3-4,5,5-trimethylcyclopentenylacetyl-CoA 1,2-monooxygenase, OTEMO) is an enzyme with systematic name ((1R)-2,2,3-trimethyl-5-oxocyclopent-3-enyl)acetyl-CoA,NADPH:oxygen oxidoreductase (1,5-lactonizing). This enzyme catalyses the following chemical reaction
L-tryptophan—pyruvate aminotransferase is an enzyme with systematic name L-tryptophan:pyruvate aminotransferase. This enzyme catalyses the following chemical reaction

The flavin-containing monooxygenase (FMO) protein family specializes in the oxidation of xeno-substrates in order to facilitate the excretion of these compounds from living organisms. These enzymes can oxidize a wide array of heteroatoms, particularly soft nucleophiles, such as amines, sulfides, and phosphites. This reaction requires an oxygen, an NADPH cofactor, and an FAD prosthetic group. FMOs share several structural features, such as a NADPH binding domain, FAD binding domain, and a conserved arginine residue present in the active site. Recently, FMO enzymes have received a great deal of attention from the pharmaceutical industry both as a drug target for various diseases and as a means to metabolize pro-drug compounds into active pharmaceuticals. These monooxygenases are often misclassified because they share activity profiles similar to those of cytochrome P450 (CYP450), which is the major contributor to oxidative xenobiotic metabolism. However, a key difference between the two enzymes lies in how they proceed to oxidize their respective substrates; CYP enzymes make use of an oxygenated heme prosthetic group, while the FMO family utilizes FAD to oxidize its substrates.

Camalexin (3-thiazol-2-yl-indole) is a simple indole alkaloid found in the plant Arabidopsis thaliana and other crucifers. The secondary metabolite functions as a phytoalexin to deter bacterial and fungal pathogens.










