
Deep-sea fish are fish that live in the darkness below the sunlit surface waters, that is below the epipelagic or photic zone of the sea. The lanternfish is, by far, the most common deep-sea fish. Other deep-sea fishes include the flashlight fish, cookiecutter shark, bristlemouths, anglerfish, viperfish, and some species of eelpout.

Stomiiformes is an order of deep-sea ray-finned fishes of very diverse morphology. It includes, for example, dragonfishes, lightfishes, loosejaws, marine hatchetfishes and viperfishes. The order contains 4 families with more than 50 genera and at least 410 species. As usual for deep-sea fishes, there are few common names for species of the order, but the Stomiiformes as a whole are often called dragonfishes and allies or simply stomiiforms.
The mesopelagiczone, also known as the middle pelagic or twilight zone, is the part of the pelagic zone that lies between the photic epipelagic and the aphotic bathypelagic zones. It is defined by light, and begins at the depth where only 1% of incident light reaches and ends where there is no light; the depths of this zone are between approximately 200 to 1,000 meters below the ocean surface.

The marine hatchetfishes or deep-sea hatchetfishes as well as the related bottlelights, pearlsides and constellationfishes are small deep-sea ray-finned fish of the stomiiform family Sternoptychidae. They are not closely related to and should not be confused with the freshwater hatchetfishes, which are teleosts in the characiform family Gasteropelecidae. The Sternoptychidae have 10 genera and about 70 species altogether.

The Gonostomatidae are a family of mesopelagic marine fish, commonly named bristlemouths, lightfishes, or anglemouths. It is a relatively small family, containing only eight known genera and 32 species. However, bristlemouths make up for their lack of diversity with relative abundance, numbering in the hundreds of trillions to quadrillions. The genus Cyclothone is thought to be one of the most abundant vertebrate genera in the world.

Lanternfish are small mesopelagic fish of the large family Myctophidae. One of two families in the order Myctophiformes, the Myctophidae are represented by 246 species in 33 genera, and are found in oceans worldwide. Lanternfishes are aptly named after their conspicuous use of bioluminescence. Their sister family, the Neoscopelidae, are much fewer in number but superficially very similar; at least one neoscopelid shares the common name "lanternfish": the large-scaled lantern fish, Neoscopelus macrolepidotus.

The Pacific viperfish, is a predatory deep-sea fish found in the North Pacific. It is reported as being either mesopelagic or bathypelagic, with diel vertical migration to shallower waters. The Pacific viperfish is one of the nine different species that belong to the genus Chauliodus, the viperfish. The Pacific viperfish tend to be the largest of the species, typically reaching lengths of up to 1 foot and are considered an example of deep-sea gigantism. The length-weight relationship of the pacific viperfish varies with sex with females tending to be longer and heavier than males.

Pelagic fish live in the pelagic zone of ocean or lake waters—being neither close to the bottom nor near the shore—in contrast with demersal fish that live on or near the bottom, and reef fish that are associated with coral reefs.

A viperfish is any species of marine fish in the genus Chauliodus. Viperfishes are mostly found in the mesopelagic zone and are characterized by long, needle-like teeth and hinged lower jaws. A typical viperfish grows to lengths of 30 cm (12 in). Viperfishes undergo diel vertical migration and are found all around the world in tropical and temperate oceans. Viperfishes are capable of bioluminescence and possess photophores along the ventral side of their body, likely used to camouflage them by blending in with the less than 1% of light that reaches to below 200 meters depth.

The bluntsnout smooth-head, black slickhead, Cope's bluntsnout smooth-head, or Atlantic gymnast, Xenodermichthys copei, is a slickhead of the genus Xenodermichthys, found in the Atlantic, Indian, and Pacific oceans, and the Tasman Sea, at depths of 100 to 2,600 m. This species grows to a length of 31 centimetres (12 in) TL.

Malacosteus niger, commonly known as the black dragon fish, is a species of deep-sea fish. Some additional common names for this species include: northern stoplight loosejaw, lightless loosejaw, black loosejaw, and black hinged-head. It belongs to the family Stomiidae, or dragonfishes. It is among the top predators of the open mesopelagic zone. M. niger is a circumglobal species, which means that it inhabits waters ranging from the tropics to the subarctics. Not many studies have been conducted on its feeding habits, but recent research suggests that M. niger primarily feed on calanoid copepods which is a form of zooplankton. Indeed, it appears that M. niger primarily prey on zooplankton despite its apparent morphological adaptations for the consumption of relatively large prey. Another unique adaptation for this species is its ability to produce both red and blue bioluminescence. Most mesopelagic species aren't capable of producing red bioluminescence. This is advantageous because most other species cannot perceive red light, therefore allowing M. niger to camouflage part of itself to its prey and predators.

Sloane's viperfish, Chauliodus sloani, is a predatory mesopelagic dragonfish found in waters across the world. The species was first described by German scientists Marcus Elieser Bloch and Johann Gottlob Schneider in their 1801 book Systema ichthyologiae: iconibus CX illustratum, volume 1. Female C. sloani reach maturity between 133 and 191 mm, while males likely reach maturity at slightly smaller body lengths. It has two rows of photophores along its ventral side. It is believed that C. sloani can adjust the intensity of bioluminescence of the ventral photophores to camouflage itself from predators that might see its shadow from below.

The blackbelly lanternshark or lucifer shark is a shark of the family Etmopteridae found around the world in tropical and temperate seas at depths between 150 and 1,250 meters – the mesopelagic zone. Compared to other mesopelagic fish predators and invertebrates, the blackbelly lanternshark is thought to reside in shallower, more southern waters. E. lucifer can reach up to 47 centimeters in length and consumes mesopelagic cephalopods, fish, and crustaceans. Blackbelly lanternsharks are bioluminescent, using hormone controlled mechanisms to emit light through ventral photogenic organs called photophores and are presumed to be ovoviviparous. The blackbelly lanternshark has been classified as "Not Threatened" within the New Zealand Threat Classification System.

Diel vertical migration (DVM), also known as diurnal vertical migration, is a pattern of movement used by some organisms, such as copepods, living in the ocean and in lakes. The adjective "diel" comes from Latin: diēs, lit. 'day', and refers to a 24-hour period. The migration occurs when organisms move up to the uppermost layer of the water at night and return to the bottom of the daylight zone of the oceans or to the dense, bottom layer of lakes during the day. DVM is important to the functioning of deep-sea food webs and the biologically-driven sequestration of carbon.
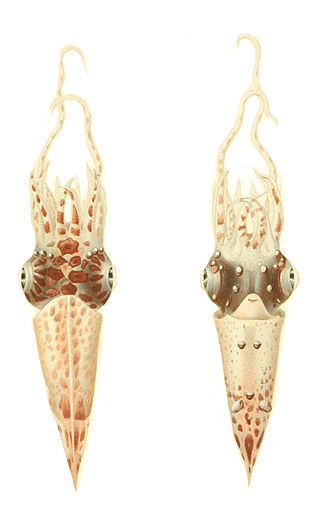
Pterygioteuthis giardi is a species of squid in the family Pyroteuthidae. It is known as the roundear enope squid. The specific name honors the French zoologist and marine biologist Alfred Mathieu Giard (1846-1908).

Todaropsis eblanae, also known as the lesser flying squid, is a species of short finned squid in the monotypic genus Todaropsis of the family Ommastrephidae.

Histioteuthis heteropsis, known as the strawberry squid, is a species of small cock-eyed squid. The scientific nomenclature of these squid stems from their set of differently sized eyes, one being small and blue and the other being large and yellow. It is thought that the large eye is used to see objects against dim light, while the smaller eye is more able to view bioluminescent light sources. The squid's vernacular name arose due to its rich red skin pigmentation and the presence of photophores along its body, making it appear like a strawberry with seeds.

Ornithoteuthis antillarum, the Atlantic bird squid, is a species of flying squid from the family Ommastrephidae which is found in the warmer waters of the Atlantic Ocean. This species is an important component of the diet of many species of fish and of cetaceans. It is taken as a bycatch in fisheries but has the potential to be commercially important if appropriate fishing methods can be developed.
Nanomia bijuga, first described by Stefano Delle Chiaje in 1844 and originally named Physsophora bijuga, is a species of mesopelagic siphonophore in the family Agalmatidae As with all members of the siphonophorae order, it is a colonial organism composed of individual zooids. N. bijuga has a fairly broad distribution, and has been observed in the coastal waters off of North America and Europe. The species has been found to occupy both epipelagic and mesopelagic depths. They utilize specialized swimming zooids for both propulsion and escape behaviors. Similar to other siphonophores, Nanomia bijuga employ stinging tentacles for hunting and defense. They primarily feed on small crustaceans, especially krill.
A micronekton is a group of organisms of 2 to 20 cm in size which are able to swim independently of ocean currents. The word 'nekton' is derived from the Greek νήκτον, translit. nekton, meaning "to swim", and was coined by Ernst Haeckel in 1890.




















