
Ascaris lumbricoides is a large parasitic roundworm of the genus Ascaris. It is the most common parasitic worm in humans. An estimated 807 million–1.2 billion people are infected with A. lumbricoides worldwide. People living in tropical and subtropical countries are at greater risk of infection. Infection by Ascaris lumbricoides is known as ascariasis.
Toxocariasis is an illness of humans caused by the dog roundworm and, less frequently, the cat roundworm. These are the most common intestinal roundworms of dogs, coyotes, wolves and foxes and domestic cats, respectively. Humans are among the many "accidental" or paratenic hosts of these roundworms.

Gnathostomiasis, also known as larva migrans profundus, is the human infection caused by the nematode Gnathostoma spinigerum and/or Gnathostoma hispidum, which infects vertebrates.

Baylisascaris is a genus of roundworms that infect more than fifty animal species.

Anisakis is a genus of parasitic nematodes that have life cycles involving fish and marine mammals. They are infective to humans and cause anisakiasis. People who produce immunoglobulin E in response to this parasite may subsequently have an allergic reaction, including anaphylaxis, after eating fish infected with Anisakis species.

Dipylidium caninum, also called the flea tapeworm, double-pored tapeworm, or cucumber tapeworm is a cyclophyllid cestode that infects organisms afflicted with fleas and canine chewing lice, including dogs, cats, and sometimes human pet-owners, especially children.

Ascaris suum, also known as the large roundworm of pig, is a parasitic nematode that causes ascariasis in pigs. While roundworms in pigs and humans are today considered as two species with different hosts, cross-infection between humans and pigs is possible; some researchers have thus argued they are the same species. Ascariasis is associated with contact to pigs and pig manure in Denmark.

The Toxocaridae are a zoonotic family of parasitic nematodes that infect canids and felids and which cause toxocariasis in humans. The worms are unable to reproduce in humans.

Eucestoda, commonly referred to as tapeworms, is the larger of the two subclasses of flatworms in the class Cestoda. Larvae have six posterior hooks on the scolex (head), in contrast to the ten-hooked Cestodaria. All tapeworms are endoparasites of vertebrates, living in the digestive tract or related ducts. Examples are the pork tapeworm with a human definitive host, and pigs as the secondary host, and Moniezia expansa, the definitive hosts of which are ruminants.
Spirometra erinaceieuropaei is a parasitic tapeworm that infects domestic animals and humans. The medical term for this infection in humans and other animals is sparganosis. Morphologically, these worms are similar to other worms in the genus Spirometra. They have a long body consisting of three sections: the scolex, the neck, and the strobilia. They have a complex life cycle that consists of three hosts, and can live in varying environments and bodily tissues. Humans can contract this parasite in three main ways. Historically, humans are considered a paratenic host; however, the first case of an adult S. erinaceieuropaei infection in humans was reported in 2017. Spirometra tapeworms exist worldwide and infection is common in animals, but S. erinaceieuropaei infections are rare in humans. Treatment for infection typically includes surgical removal and anti-worm medication.

Ancylostoma duodenale is a species of the roundworm genus Ancylostoma. It is a parasitic nematode worm and commonly known as the Old World hookworm. It lives in the small intestine especially the jejunum of definitive hosts, generally humans, where it is able to mate and mature. Ancylostoma duodenale and Necator americanus are the two human hookworm species that are normally discussed together as the cause of hookworm infection. They are dioecious. Ancylostoma duodenale is abundant throughout the world, including Southern Europe, North Africa, India, China, Southeast Asia, some areas in the United States, the Caribbean, and South America.

The Strongylida suborder includes many of the important nematodes found in the gastrointestinal tracts of ruminants, horses, and swine, as well as the lungworms of ruminants and the hookworms of dogs and cats.

Toxocara canis is a worldwide-distributed helminth parasite that primarily infects dogs and other canids, but can also infect other animals including humans. The name is derived from the Greek word toxon 'bow, quiver' and the Latin word caro 'flesh'. T. canis live in the small intestine of the definitive host. This parasite is very common in puppies and somewhat less common in adult dogs. In adult dogs, infection is usually asymptomatic but may be characterized by diarrhea. By contrast, untreated infection with Toxocara canis can be fatal in puppies, causing diarrhea, vomiting, pneumonia, enlarged abdomen, flatulence, poor growth rate, and other complications.

The Anisakidae are a family of intestinal nematodes (roundworms). The larvae of these worms can cause anisakiasis when ingested by humans, in raw or insufficiently cooked fish.

Baylisascaris procyonis, also known by the common name raccoon roundworm, is a roundworm nematode, found ubiquitously in raccoons, the definitive hosts. It is named after H. A. Baylis, who studied them in the 1920s–30s, and Greek askaris. Baylisascaris larvae in paratenic hosts can migrate, causing larva migrans. Baylisascariasis as the zoonotic infection of humans is rare, though extremely dangerous due to the ability of the parasite's larvae to migrate into brain tissue and cause damage. Concern for human infection has been increasing over the years due to the urbanization of rural areas, resulting in the increase in proximity and potential human interaction with raccoons.
Gnathostoma hispidum is a nematode (roundworm) that infects many vertebrate animals including humans. Infection of Gnathostoma hispidum, like many species of Gnathostoma causes the disease gnathostomiasis due to the migration of immature worms in the tissues.
Eustrongylidosis is a parasitic disease that mainly affects wading birds worldwide; however, the parasite's complex, indirect lifecycle involves other species, such as aquatic worms and fish. Moreover, this disease is zoonotic, which means the parasite can transmit disease from animals to humans. Eustrongylidosis is named after the causative agent Eustrongylides, and typically occurs in eutrophicated waters where concentrations of nutrients and minerals are high enough to provide ideal conditions for the parasite to thrive and persist. Because eutrophication has become a common issue due to agricultural runoff and urban development, cases of eustrongylidosis are becoming prevalent and hard to control. Eustrongylidosis can be diagnosed before or after death by observing behavior and clinical signs, and performing fecal flotations and necropsies. Methods to control it include preventing eutrophication and providing hosts with uninfected food sources in aquaculture farms. Parasites are known to be indicators of environmental health and stability, so should be studied further to better understand the parasite's lifecycle and how it affects predator-prey interactions and improve conservation efforts.

Contracaecum is a genus of parasitic nematodes from the family Anisakidae. These nematodes are parasites of warm-blooded, fish eating animals, i.e. mammals and birds, as sexually mature adults. The eggs and the successive stages of their larvae use invertebrates and increasing size classes of fishes as intermediate hosts. It is the only genus in the family Anisakidae which can infect terrestrial, marine and freshwater animals.

Cat worm infections, the infection of cats (Felidae) with parasitic worms, occur frequently. Most worm species occur worldwide in both domestic and other cats, but there are regional, species and lifestyle differences in the frequency of infestation. According to the classification of the corresponding parasites in the zoological system, infections can be divided into those caused by nematode and flatworms - in the case of the latter, mainly cestoda and trematoda - while other strains are of no veterinary significance. While threadworms usually do not require an intermediate host for their reproduction, the development cycle of flatworms always proceeds via alternate hosts.
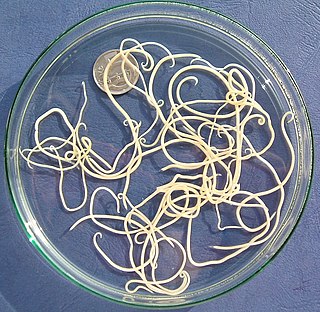
Nematode infection in dogs - the infection of dogs with parasitic nemamotodes - are, along with tapeworm infections and infections with protozoa, frequent parasitoses in veterinary practice. Nematodes, as so-called endoparasites, colonize various internal organs - most of them the digestive tract - and the skin. To date, about 30 different species of nematode have been identified in domestic dogs; they are essentially also found in wild dog species. However, the majority of them often cause no or only minor symptoms of disease in adult animals. The infection therefore does not necessarily have to manifest itself in a worm disease (helminthosis). For most nematodes, an infection can be detected by examining the feces for eggs or larvae. Roundworm infection in dogs and the hookworm in dogs is of particular health significance in Central Europe, as they can also be transmitted to humans (zoonosis). Regular deworming can significantly reduce the frequency of infection and thus the risk of infection for humans and dogs.
















