Related Research Articles
The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Neptunium is a chemical element; it has symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar System, which uranium is named after. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. Like all actinides, it is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.

Neptunium(IV) oxide, or neptunium dioxide, is a radioactive, olive green cubic crystalline solid with the formula NpO2. It emits both α- and γ-particles.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is an orange volatile crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.

Neptunocene, Np(C8H8)2, is an organoneptunium compound composed of a neptunium atom sandwiched between two cyclooctatetraenide (COT2-) rings. As a solid it has a dark brown/red colour but it appears yellow when dissolved in chlorocarbons, in which it is sparingly soluble. The compound is quite air-sensitive.
Neptunium(III) fluoride or neptunium trifluoride is a salt of neptunium and fluorine with the formula NpF3.

Neptunium(IV) fluoride or neptunium tetrafluoride is a inorganic compound with the formula NpF4. It is a green salt and is isostructural with UF4.
Neptunium(V) fluoride or neptunium pentafluoride is a chemical compound of neptunium and fluorine with the formula NpF5.

Neptunium (IV) oxalate is an inorganic compound, a salt of neptunium and oxalic acid with the chemical formula Np(C2O4)2. The compound is slightly soluble in water, forms crystalline hydrates—green crystals.
Neptunium arsenide is a binary inorganic compound of neptunium and arsenic with the chemical formula NpAs. The compound forms crystals.
Neptunium diarsenide is a binary inorganic compound of neptunium and arsenic with the chemical formula NpAs
2. The compound forms crystals.
Neptunium silicide is a binary inorganic compound of neptunium and silicon with the chemical formula NpSi
2. The compound forms crystals and does not dissolve in water.
Neptunium(IV) nitrate is an inorganic compound, a salt of neptunium and nitric acid with the chemical formula Np(NO3)4. The compound forms gray crystals, dissolves in water, and forms crystal hydrates.
Neptunium(VII) oxide-hydroxide is a chemical compound which has neptunium in its highest oxidation state of +7. This compound reacts with basic salts such as potassium hydroxide to form neptunates (NpO53-) and water.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.
Neptunium(III) phosphide is a binary inorganic compound of neptunium metal and phosphorus with the chemical formula NpP.
Neptunium(IV) phosphide is a binary inorganic compound of neptunium metal and phosphorus with the chemical formula Np3P4.

Neptunium tetrachloride is a binary inorganic compound of neptunium metal and chlorine with the chemical formula NpCl4.
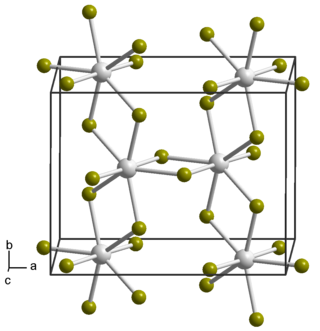
Neptunium tetrabromide is a binary inorganic compound of neptunium metal and bromine with the chemical formula NpBr4.
References
- ↑ Lemire, Robert J. (26 August 2001). Chemical Thermodynamics of Neptunium and Plutonium. Elsevier. p. 201. ISBN 978-0-444-50379-4 . Retrieved 28 March 2024.
- ↑ Erdos, Paul (6 December 2012). The Physics of Actinide Compounds. Springer Science & Business Media. p. 37. ISBN 978-1-4613-3581-8 . Retrieved 28 March 2024.
- ↑ Comprehensive Nuclear Materials. Elsevier. 22 July 2020. p. 187. ISBN 978-0-08-102866-7 . Retrieved 28 March 2024.
- ↑ Advances in Inorganic Chemistry and Radiochemistry. Academic Press. 1968. p. 205. ISBN 978-0-08-057860-6 . Retrieved 28 March 2024.
- ↑ Donnay, Joseph Désiré Hubert (1972). Crystal Data: Organic compounds. National Bureau of Standards. p. 3. Retrieved 28 March 2024.