
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard-brittle, grayish-white metalloid in the carbon group, chemically similar to its group neighbors silicon and tin. Pure germanium is an indirect semiconductor with an appearance similar to elemental silicon. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.

Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution.

Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.
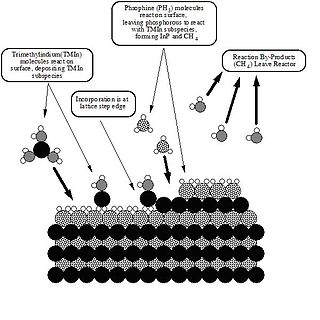
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures. As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as Light-emitting diodes. It was invented in 1968 at North American Aviation Science Center by Harold M. Manasevit.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
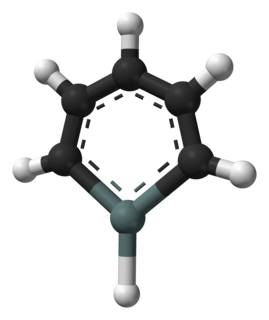
Stannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry, but has not been isolated.

Germabenzene (C5H6Ge) is the parent representative of a group of chemical compounds containing in their molecular structure a benzene ring with a carbon atom replaced by a germanium atom. Germabenzene itself has been studied theoretically, and synthesized with a bulky 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl or Tbt group. Also, stable naphthalene derivatives do exist in the laboratory such as the 2-germanaphthalene-containing substance represented below. The germanium to carbon bond in this compound is shielded from potential reactants by a Tbt group. This compound is aromatic just as the other carbon group representatives silabenzene and stannabenzene.

Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
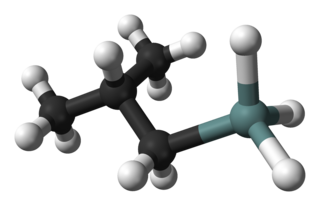
Isobutylgermane (IBGe, Chemical formula: (CH3)2CHCH2GeH3, is an organogermanium compound. It is a colourless, volatile liquid that is used in MOVPE (Metalorganic Vapor Phase Epitaxy) as an alternative to germane. IBGe is used in the deposition of Ge films and Ge-containing thin semiconductor films such as SiGe in strained silicon application, and GeSbTe in NAND Flash applications.

Trimethyltin chloride is an organotin compound with the formula (CH3)3SnCl. It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis.

Tetraethylgermanium (common name tetraethyl germanium), abbreviated TEG, is an organogermanium compound with the formula (CH3CH2)4Ge. Tetraethylgermanium is an important chemical compound used in vapour deposition of germanium which is in a tetrahedral shape.

Disilyne is a silicon hydride with the formula Si
2H
2. Several isomers are possible, but none are sufficiently stable to be of practical value. Substituted disilynes contain a formal silicon–silicon triple bond and as such are sometimes written R2Si2 (where R is a substituent group). They are the silicon analogues of alkynes.

Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms.
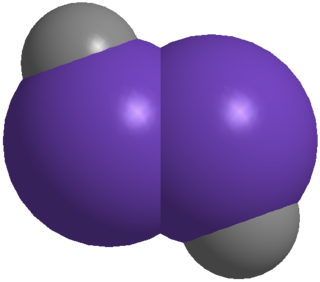
Digermynes are a class of compounds that are regarded as the heavier digermanium analogues of alkynes. The parent member of this entire class is HGeGeH, which has only been characterized computationally, but has revealed key features of the whole class. Because of the large interatomic repulsion between two Ge atoms, only kinetically stabilized digermyne molecules can be synthesized and characterized by utilizing bulky protecting groups and appropriate synthetic methods, for example, reductive coupling of germanium(II) halides.
Germanium(II) hydrides, also called germylene hydrides, are a class of Group 14 compounds consisting of low-valent germanium and a terminal hydride. They are also typically stabilized by an electron donor-acceptor interaction between the germanium atom and a large, bulky ligand.

A trivalent group 14 radical (also known as a trivalent tetrel radical) is a molecule that contains a group 14 element (E = C, Si, Ge, Sn, Pb) with three bonds and a free radical, having the general formula of R3E•. Such compounds can be categorized into three different types, depending on the structure (or equivalently the orbital in which the unpaired electron resides) and the energetic barrier to inversion. A molecule that remains rigidly in a pyramidal structure has an electron in a sp3 orbital is denoted as Type A. A structure that is pyramidal, but flexible, is denoted as Type B. And a planar structure with an electron that typically would reside in a pure p orbital is denoted as Type C. The structure of such molecules has been determined by probing the nature of the orbital that the unpaired electron resides in using spectroscopy, as well as directly with X-ray methods. Trivalent tetrel radicals tend to be synthesized from their tetravalent counterparts (i.e. R3EY where Y is a species that will dissociate).
Germanium dichloride dioxane is a chemical compound with the formula GeCl2(C4H8O2), where C4H8O2 is 1,4-dioxane. It is a white solid. The compound is notable as a source of Ge(II), which contrasts with the pervasiveness of Ge(IV) compounds. This dioxane complex represents a well-behaved form of germanium dichloride.

















