
Protoporphyrinogen oxidase or protox is an enzyme that in humans is encoded by the PPOX gene.

In biochemistry, mixed acid fermentation is the metabolic process by which a six-carbon sugar is converted into a complex and variable mixture of acids. It is an anaerobic (non-oxygen-requiring) fermentation reaction that is common in bacteria. It is characteristic for members of the Enterobacteriaceae, a large family of Gram-negative bacteria that includes E. coli.

Protoporphyrinogen IX is an organic chemical compound which is produced along the synthesis of porphyrins, a class of critical biochemicals that include hemoglobin and chlorophyll. It is a direct precursor of protoporphyrin IX.
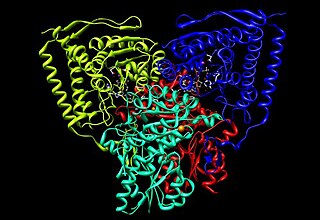
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.
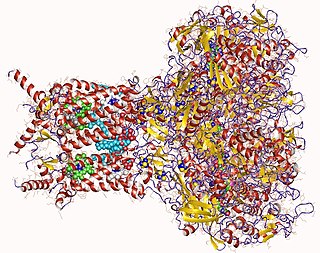
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase (EC 1.17.1.9) or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase (EC 1.2.2.1). This family of enzymes has attracted attention as inspiration or guidance on methods for the carbon dioxide fixation, relevant to global warming.

In enzymology, a shikimate dehydrogenase (EC 1.1.1.25) is an enzyme that catalyzes the chemical reaction
In enzymology, an erythrose-4-phosphate dehydrogenase (EC 1.2.1.72) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-phosphoerythronate dehydogenase (EC 1.1.1.290) is an enzyme that catalyzes the chemical reaction
In enzymology, a glycerol-3-phosphate dehydrogenase [NAD(P)+] (EC 1.1.1.94) is an enzyme that catalyzes the chemical reaction
In enzymology, a coproporphyrinogen dehydrogenase (EC 1.3.99.22) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetyl-gamma-glutamyl-phosphate reductase (EC 1.2.1.38) is an enzyme that catalyzes the chemical reaction

Cytochrome c nitrite reductase (ccNiR) is a bacterial enzyme that catalyzes the six electron reduction of nitrite to ammonia; an important step in the biological nitrogen cycle. The enzyme catalyses the second step in the two step conversion of nitrate to ammonia, which allows certain bacteria to use nitrite as a terminal electron acceptor, rather than oxygen, during anaerobic conditions. During this process, ccNiR draws electrons from the quinol pool, which are ultimately provided by a dehydrogenase such as formate dehydrogenase or hydrogenase. These dehydrogenases are responsible for generating a proton motive force.

Isochorismate synthase ( EC 5.4.4.2) is an isomerase enzyme that catalyzes the first step in the biosynthesis of vitamin K2 (menaquinone) in Escherichia coli.
2-Succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase, also known as SHCHC synthase is encoded by the menH gene in Escherichia coli and functions in the synthesis of vitamin K. The specific step in the synthetic pathway that SHCHC synthase catalyzes is the conversion of 5-enolpyruvoyl-6-hydroxy-2-succinylcyclohex-3-ene-1-carboxylate to (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate and pyruvate.
Quinate/shikimate dehydrogenase (EC 1.1.1.282, YdiB) is an enzyme with systematic name L-quinate:NAD(P)+ 3-oxidoreductase. This enzyme catalyses the following chemical reaction

Fumarate reductase (quinol) (EC 1.3.5.4, QFR,FRD, menaquinol-fumarate oxidoreductase, quinol:fumarate reductase) is an enzyme with systematic name succinate:quinone oxidoreductase. This enzyme catalyzes the following chemical reaction:

Phytoene desaturase (lycopene-forming) are enzymes found in archaea, bacteria and fungi that are involved in carotenoid biosynthesis. They catalyze the conversion of colorless 15-cis-phytoene into a bright red lycopene in a biochemical pathway called the poly-trans pathway. The same process in plants and cyanobacteria utilizes four separate enzymes in a poly-cis pathway.
Pyruvate dehydrogenase (quinone) (EC 1.2.5.1, pyruvate dehydrogenase, pyruvic dehydrogenase, pyruvic (cytochrome b1) dehydrogenase, pyruvate:ubiquinone-8-oxidoreductase, pyruvate oxidase (ambiguous)) is an enzyme with systematic name pyruvate:ubiquinone oxidoreductase. This enzyme catalyses the following chemical reaction
3-oxo-5,6-dehydrosuberyl-CoA semialdehyde dehydrogenase (EC 1.17.1.7, paaZ (gene)) is an enzyme with systematic name 3-oxo-5,6-dehydrosuberyl-CoA semialdehyde:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
Demethylmenaquinone methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:demethylmenaquinone methyltransferase. This enzyme catalyses the following chemical reaction










