This article needs to be updated.(July 2023) |
| Soil-transmitted helminthiasis | |
|---|---|
| Other names | STH |
 | |
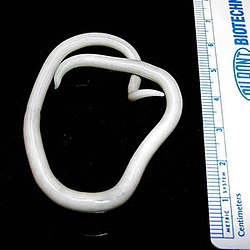
| Adult ascaris worms being removed from the bile duct of a patient in South Africa | |
Soil-transmitted helminthiasis is a type of worm infection (helminthiasis) caused by different species of roundworms. It is caused specifically by worms transmitted through soil contaminated with faecal matter and are known as soil-transmitted helminths. Three types of soil-transmitted helminthiasis can be distinguished: ascariasis, hookworm infection and whipworm infection. These three types of infection are therefore caused by the large roundworm A. lumbricoides, the hookworms Necator americanus or Ancylostoma duodenale and by the whipworm Trichuris trichiura .
Contents
- Types
- Ascariasis
- Hookworm disease
- Trichuriasis
- Signs and symptoms
- General
- Malnutrition
- Diagnosis
- Prevention
- Treatment
- Periodical mass administration of anthelmintics
- Drugs for those with other diseases
- Surgical intervention
- Epidemiology
- Regions
- Infection estimates
- Deaths
- References
- External links
It has become the most common parasitic disease of humans worldwide. [1] Approximately two billion people (about a fourth of global population) are infected as of the latest estimate, and four billion at risk, surpassing even the all-time most prevalent parasitic disease, malaria. [2] The largest numbers of cases occur in impoverished rural areas of Sub-Saharan Africa, Latin America, Southeast Asia, and China. [3] Its main cause, like for many types of helminth infections, is lack of sanitation, such as the practice of open defecation, lack of hygiene such as hand washing and walking barefoot on contaminated soil. [4] [5] [6] It is regarded as one of the world's most important causes of intellectual and physical retardation. [7]
The helminthic disease is so named because the infection is transmitted through ingestion of the nematode eggs in the soil, which is contaminated through excrements. Therefore, the disease is most prevalent in warm and moist climates where sanitation and hygiene are poor and waters are unsafe, including the temperate zones during warmer months. STH is categorised among neglected tropical diseases because it inflicts tremendous disability and suffering, which can be clinically treated and relatively easily prevented (primarily through improved sanitation), yet negligible attention has been given for many years. [8] It is now among the target diseases of London Declaration on Neglected Tropical Diseases (launched on 30 January 2012) to be controlled/eradicated by 2020. [9]
Simple prevention and control strategies are access to improved sanitation, public awareness of personal hygiene, and health education.