| trans-2-enoyl-CoA reductase (NADPH) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.3.1.38 | ||||||||
| CAS no. | 77649-64-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
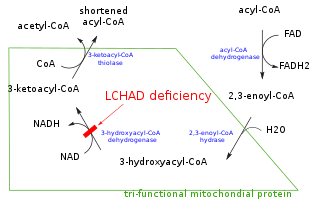
In enzymology, a trans-2-enoyl-CoA reductase (NADPH) (EC 1.3.1.38) is an enzyme that catalyzes the chemical reaction
- trans-2,3-dehydroacyl-CoA + NADPH + H+ acyl-CoA + NADP+
Thus, the three substrates of this enzyme are trans-2,3-dehydroacyl-CoA, NADPH, and H+, whereas its two products are acyl-CoA and NADP+.
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-CH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is acyl-CoA:NADP+ trans-2-oxidoreductase. Other names in common use include NADPH-dependent trans-2-enoyl-CoA reductase, reductase, trans-enoyl coenzyme A, and trans-2-enoyl-CoA reductase (NADPH). This enzyme participates in fatty acid elongation in mitochondria and polyunsaturated fatty acid biosynthesis.