This article needs additional citations for verification .(September 2016) |
| Transitional cell carcinoma | |
|---|---|
| Other names | Urothelial carcinoma |
 | |
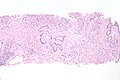
| Histopathology of transitional carcinoma of the urinary bladder. Transurethral biopsy. Hematoxylin and eosin stain. | |
| Specialty | Oncology |
Transitional cell carcinoma is a type of cancer that arises from the transitional epithelium, a tissue lining the inner surface of these hollow organs. [1] It typically occurs in the urothelium of the urinary system; in that case, it is also called urothelial carcinoma. It is the most common type of bladder cancer and cancer of the ureter, urethra, and urachus. Symptoms of urothelial carcinoma in the bladder include hematuria (blood in the urine). Diagnosis includes urine analysis and imaging of the urinary tract (cystoscopy).
Contents
- Signs and symptoms
- Causes
- Growth and spread
- Diagnosis
- Classification
- Treatment
- Localized/early transitional cell carcinomas of bladder
- Advanced or metastatic transitional cell carcinomas
- Prostate
- See also
- References
- External links
It accounts for 95% of bladder cancer cases and bladder cancer is in the top 10 most common malignancy disease in the world and is associated with approximately 200,000 deaths per year in the United States alone. [2] [3] It is the second most common type of kidney cancer, but accounts for only five to 10 percent of all primary renal malignant tumors. [4] Men and older people have a higher rate of urothelial carcinomas. Other risk factors include smoking and exposure to aromatic amines. [5]
Treatment approaches depend on the stage and spread of the tumour. Tumour removal (resection), chemotherapy and chemoradiation may be indicated. Immunotherapy with immune check point inhibitor medications may also be suggested. [5]