Related Research Articles

Alcoholic liver disease (ALD), also called alcohol-related liver disease (ARLD), is a term that encompasses the liver manifestations of alcohol overconsumption, including fatty liver, alcoholic hepatitis, and chronic hepatitis with liver fibrosis or cirrhosis.

Budd–Chiari syndrome is a very rare condition, affecting one in a million adults. The condition is caused by occlusion of the hepatic veins that drain the liver. The symptoms are non-specific and vary widely, but it may present with the classical triad of abdominal pain, ascites, and liver enlargement. It is usually seen in younger adults, with the median age at diagnosis between the ages of 35 to 40, and it has a similar incidence in males and females. The syndrome can be fulminant, acute, chronic, or asymptomatic. Subacute presentation is the most common form.
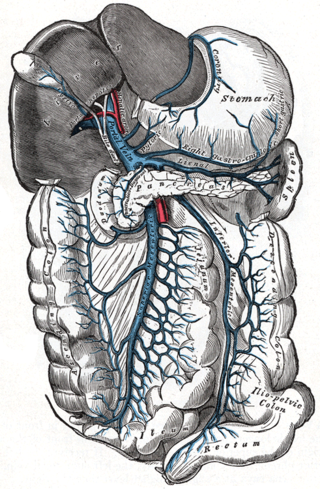
Portal hypertension is abnormally increased portal venous pressure – blood pressure in the portal vein and its branches, that drain from most of the intestine to the liver. Portal hypertension is defined as a hepatic venous pressure gradient greater than 5 mmHg. Cirrhosis is the most common cause of portal hypertension; other, less frequent causes are therefore grouped as non-cirrhotic portal hypertension. When it becomes severe enough to cause symptoms or complications, treatment may be given to decrease portal hypertension itself or to manage its complications.
In medicine, specifically gastroenterology, the Child–Pugh score is used to assess the prognosis of chronic liver disease, mainly cirrhosis. Although it was originally used to predict mortality during surgery, it is now used to determine the prognosis, as well as the required strength of treatment and the necessity of liver transplantation.

Acute liver failure is the appearance of severe complications rapidly after the first signs of liver disease, and indicates that the liver has sustained severe damage. The complications are hepatic encephalopathy and impaired protein synthesis. The 1993 classification defines hyperacute as within 1 week, acute as 8–28 days, and subacute as 4–12 weeks; both the speed with which the disease develops and the underlying cause strongly affect outcomes.

Hepatorenal syndrome is a life-threatening medical condition that consists of rapid deterioration in kidney function in individuals with cirrhosis or fulminant liver failure. HRS is usually fatal unless a liver transplant is performed, although various treatments, such as dialysis, can prevent advancement of the condition.

Liver biopsy is the biopsy from the liver. It is a medical test that is done to aid diagnosis of liver disease, to assess the severity of known liver disease, and to monitor the progress of treatment.

Transjugular intrahepatic portosystemic shunt is an artificial channel within the liver that establishes communication between the inflow portal vein and the outflow hepatic vein. It is used to treat portal hypertension which frequently leads to intestinal bleeding, life-threatening esophageal bleeding and the buildup of fluid within the abdomen (ascites).
Pediatric end-stage liver disease (PELD) is a disease severity scoring system for children under 12 years of age. It is calculated from the patient's albumin, bilirubin, and international normalized ratio (INR) together with the patient's age and degree of growth failure. This score is also used by the United Network for Organ Sharing (UNOS) for prioritizing allocation of liver transplants.
The King's College Criteria or the King's College Hospital criteria were devised in 1989 to determine if there were any early indices of poor prognosis in patients with acute liver failure. Acute liver failure is defined as the onset of encephalopathy or coagulopathy within 26 weeks of a patient diagnosed with liver disease. Patients with hepatitis B acquired at birth, Wilson's disease and autoimmune hepatitis are included if their disease was identified within the past 26 weeks. These patients are very ill, and have a very high risk of dying of their illness without adequate treatment which may include liver transplantation. It is important that physicians find ways of identifying patients with acute liver failure early in their course who will do poorly, and may require liver transplantation. The King's College Criteria have consistently shown excellent operating characteristics for determining prognosis in these patients. As liver transplantation becomes a more accessible option for patients with acute liver failure, the King's College Criteria serve a role in determining which patients may require transplantation.
Hy's law is a rule of thumb that a patient is at high risk of a fatal drug-induced liver injury if given a medication that causes hepatocellular injury with jaundice. The law is based on observations by Hy Zimmerman, a major scholar of drug-induced liver injury. Some have suggested the principle be called a hypothesis or observation.
Maddrey's discriminant function (DF) is the traditional model for evaluating the severity and prognosis in alcoholic hepatitis and evaluates the efficacy of using alcoholic hepatitis steroid treatment. The Maddrey DF score is a predictive statistical model compares the subject's DF score with mortality prognosis within 30-day or 90-day scores. The subject's Maddrey DF score is determined by blood analysis.

Cirrhosis, also known as liver cirrhosis or hepatic cirrhosis, and end-stage liver disease, is the impaired liver function caused by the formation of scar tissue known as fibrosis due to damage caused by liver disease. Damage to the liver leads to repair of liver tissue and subsequent formation of scar tissue. Over time, scar tissue can replace normal functioning tissue, leading to the impaired liver function of cirrhosis. The disease typically develops slowly over months or years. Early symptoms may include tiredness, weakness, loss of appetite, unexplained weight loss, nausea and vomiting, and discomfort in the right upper quadrant of the abdomen. As the disease worsens, symptoms may include itchiness, swelling in the lower legs, fluid build-up in the abdomen, jaundice, bruising easily, and the development of spider-like blood vessels in the skin. The fluid build-up in the abdomen develop spontaneous infections. More serious complications include hepatic encephalopathy, bleeding from dilated veins in the esophagus, stomach, or intestines, and liver cancer.
FibroTest, known as FibroSure in the US, is a biomarker test that uses the results of six blood serum tests to generate a score that is correlated with the degree of liver damage in people with a variety of liver diseases. FibroTest has the same prognostic value as a liver biopsy. FibroSure uses quantitative results of five serum biochemical markers, α2-macroglobulin, haptoglobin, apolipoprotein A1, bilirubin, gamma glutamyl transpeptidase (GGT), with a patient’s age and gender to generate a measure of fibrosis and necroinflammatory activity in the liver.
The United Kingdom Model for End-Stage Liver Disease or UKELD is a medical scoring system used to predict the prognosis of patients with chronic liver disease. It is used in the United Kingdom to help determine the need for liver transplantation. It was developed from the MELD score, incorporating the serum sodium level.
A liver support system or diachysis is a type of therapeutic device to assist in performing the functions of the liver. Such systems focus either on removing the accumulating toxins, or providing additional replacement of the metabolic functions of the liver through the inclusion of hepatocytes to the device. This system is in trial to help people with acute liver failure (ALF) or acute-on-chronic liver failure.
The Lille Model is a medical modeling tool for predicting mortality in patients with alcoholic hepatitis who are not responding to steroid therapy. The model risk stratifies patients who have been receiving steroid treatment for seven days to predict who will improve and who should be considered for alternative treatment options including early referral for transplant.

MELD-Plus is a risk score to assess severity of chronic liver disease that was resulted from a collaboration between Massachusetts General Hospital and IBM. The score includes nine variables as effective predictors for 90-day mortality after a discharge from a cirrhosis-related admission. The variables include all Model for End-Stage Liver Disease (MELD)'s components, as well as sodium, albumin, total cholesterol, white blood cell count, age, and length of stay.
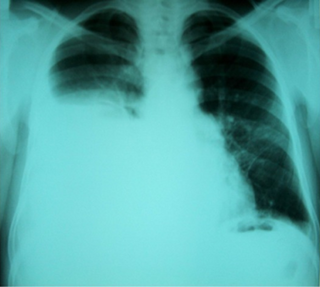
Hepatic hydrothorax is a rare form of pleural effusion that occurs in people with liver cirrhosis. It is defined as an effusion of over 500 mL in people with liver cirrhosis that is not caused by heart, lung, or pleural disease. It is found in 5-10% of people with liver cirrhosis and 2-3% of people with pleural effusions. It is much more common on the right side, with 85% of cases occurring on the right, 13% on the left, and 2% on both. Although it is most common in people with severe ascites, people with mild or no ascites have had the condition. Symptoms are not specific and mostly involve the respiratory system.
Hyperbilirubinemia is a clinical condition describing an elevation of blood bilirubin level due to the inability to properly metabolise or excrete bilirubin, a product of erythrocytes breakdown. In severe cases, it is manifested as jaundice, the yellowing of tissues like skin and the sclera when excess bilirubin deposits in them. The US records 52,500 jaundice patients annually. By definition, bilirubin concentration of greater than 3 mg/ml is considered hyperbilirubinemia, following which jaundice progressively develops and becomes apparent when plasma levels reach 20 mg/ml. Rather than a disease itself, hyperbilirubinemia is indicative of multifactorial underlying disorders that trace back to deviations from regular bilirubin metabolism. Diagnosis of hyperbilirubinemia depends on physical examination, urinalysis, serum tests, medical history and imaging to identify the cause. Genetic diseases, alcohol, pregnancy and hepatitis viruses affect the likelihood of hyperbilirubinemia. Causes of hyperbilirubinemia mainly arise from the liver. These include haemolytic anaemias, enzymatic disorders, liver damage and gallstones. Hyperbilirubinemia itself is often benign. Only in extreme cases does kernicterus, a type of brain injury, occur. Therapy for adult hyperbilirubinemia targets the underlying diseases but patients with jaundice often have poor outcomes.
References
- 1 2 Malinchoc, Michael; Kamath, Patrick S; Gordon, Fredric D; Peine, Craig J; Rank, Jeffrey; Ter Borg, Pieter C.J (2000). "A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts". Hepatology. 31 (4): 864–71. doi: 10.1053/he.2000.5852 . PMID 10733541.
- ↑ Kamath, P; Wiesner, R. H; Malinchoc, M; Kremers, W; Therneau, T. M; Kosberg, C. L; d'Amico, G; Dickson, E. R; Kim, W. R (2001). "A model to predict survival in patients with end-stage liver disease". Hepatology. 33 (2): 464–70. doi:10.1053/jhep.2001.22172. PMID 11172350. S2CID 72518575.
- 1 2 3 4 Kamath, Patrick S; Kim, W. Ray (2007). "The model for end-stage liver disease (MELD)". Hepatology. 45 (3): 797–805. doi: 10.1002/hep.21563 . PMID 17326206.
- ↑ Jung, G.E; Encke, J; Schmidt, J; Rahmel, A (2008). "Model for end-stage liver disease". Der Chirurg. 79 (2): 157–63. doi:10.1007/s00104-008-1463-4. PMID 18214398. S2CID 25562795.
- ↑ UNOS (2009-01-28). "MELD/PELD calculator documentation" (PDF). Retrieved 2010-02-21.
- 1 2 3 4 5 6 Wiesner, Russell; Edwards, Erick; Freeman, Richard; Harper, Ann; Kim, Ray; Kamath, Patrick; Kremers, Walter; Lake, John; Howard, Todd; Merion, Robert M.; Wolfe, Robert A.; Krom, Ruud (2003-01-01). "Model for end-stage liver disease (MELD) and allocation of donor livers". Gastroenterology. 124 (1): 91–96. doi:10.1053/gast.2003.50016. ISSN 0016-5085. PMID 12512033.
- ↑ Kartoun, Uri; Corey, Kathleen E; Simon, Tracey G; Zheng, Hui; Aggarwal, Rahul; Ng, Kenney; Shaw, Stanley Y (2017). "The MELD-Plus: A generalizable prediction risk score in cirrhosis". PLOS ONE. 12 (10): e0186301. Bibcode:2017PLoSO..1286301K. doi: 10.1371/journal.pone.0186301 . PMC 5656314 . PMID 29069090.
- ↑ "OPTN/UNOS Liver and Intestinal Organ Transplantation Committee Report to the Board of Directors" (PDF). hrsa.gov. June 2014. Retrieved 26 April 2023.
- ↑ Kim, WR; Biggins, SW; Kremers, WK; Wiesner, RH; Kamath, PS; Benson, JT; Edwards, E; Therneau, TM (2008). "Hyponatremia and mortality among patients on the liver-transplant waiting list". N Engl J Med. 359 (10): 1018–6. doi:10.1056/NEJMoa0801209. PMC 4374557 . PMID 18768945.
- ↑ Kartoun, Uri (2018). "Toward an accelerated adoption of data-driven findings in medicine". Medicine, Health Care and Philosophy. 22 (1): 153–157. doi:10.1007/s11019-018-9845-y. PMID 29882052. S2CID 46973857.