
In pharmacology and toxicology, a route of administration is the way by which a drug, fluid, poison, or other substance is taken into the body.
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; the terms are equivalent in the literature. Larger structures such as nucleic acids and proteins, and many polysaccharides are not small molecules, although their constituent monomers are often considered small molecules. Small molecules may be used as research tools to probe biological function as well as leads in the development of new therapeutic agents. Some can inhibit a specific function of a protein or disrupt protein–protein interactions.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
In the physical sciences, a partition coefficient (P) or distribution coefficient (D) is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solubilities of the solute in these two liquids. The partition coefficient generally refers to the concentration ratio of un-ionized species of compound, whereas the distribution coefficient refers to the concentration ratio of all species of the compound.

ADME is the four-letter abbreviation (acronym) for absorption, distribution, metabolism, and excretion, and is mainly used in fields such as pharmacokinetics and pharmacology. The four letter stands for descriptors quantifying how a given drug interacts within body over time. The term ADME was first introduced in 1960s, and has become a standard term widely used in scientific literature, teaching, drug regulations, and clinical practice.
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes including creams, foams, gels, lotions, and ointments. Many topical medications are epicutaneous, meaning that they are applied directly to the skin. Topical medications may also be inhalational, such as asthma medications, or applied to the surface of tissues other than the skin, such as eye drops applied to the conjunctiva, or ear drops placed in the ear, or medications applied to the surface of a tooth. The word topical derives from Greek τοπικόςtopikos, "of a place".
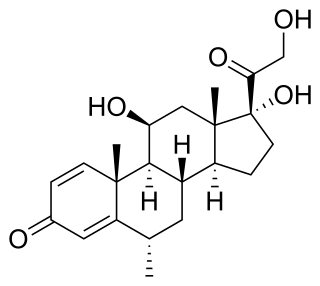
Methylprednisolone is a synthetic glucocorticoid, primarily prescribed for its anti-inflammatory and immunosuppressive effects. It is either used at low doses for chronic illnesses or used concomitantly at high doses during acute flares. Methylprednisolone and its derivatives can be administered orally or parenterally.
Sublingual, from the Latin for "under the tongue", refers to the pharmacological route of administration by which substances diffuse into the blood through tissues under the tongue.

Enteral administration is food or drug administration via the human gastrointestinal tract. This contrasts with parenteral nutrition or drug administration, which occurs from routes outside the GI tract, such as intravenous routes. Enteral administration involves the esophagus, stomach, and small and large intestines. Methods of administration include oral, sublingual, and rectal. Parenteral administration is via a peripheral or central vein. In pharmacology, the route of drug administration is important because it affects drug metabolism, drug clearance, and thus dosage. The term is from Greek enteros 'intestine'.
Skin absorption is a route by which substances can enter the body through the skin. Along with inhalation, ingestion and injection, dermal absorption is a route of exposure for toxic substances and route of administration for medication. Absorption of substances through the skin depends on a number of factors, the most important of which are concentration, duration of contact, solubility of medication, and physical condition of the skin and part of the body exposed.
Pharmaceutical formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form.
Modified-release dosage is a mechanism that delivers a drug with a delay after its administration or for a prolonged period of time or to a specific target in the body.
Dose dumping is a phenomenon of drug metabolism in which environmental factors can cause the premature and exaggerated release of a drug. This can greatly increase the concentration of a drug in the body and thereby produce adverse effects or even drug-induced toxicity.
In cell biology, ion trapping is the build-up of a higher concentration of a chemical across a cell membrane due to the pKa value of the chemical and difference of pH across the cell membrane. This results in basic chemicals accumulating in acidic bodily fluids such as the cytosol, and acidic chemicals accumulating in basic fluids.

Oral administration is a route of administration whereby a substance is taken through the mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.
Buccal administration is a topical route of administration by which drugs held or applied in the buccal area diffuse through the oral mucosa and enter directly into the bloodstream. Buccal administration may provide better bioavailability of some drugs and a more rapid onset of action compared to oral administration because the medication does not pass through the digestive system and thereby avoids first pass metabolism. Drug forms for buccal administration include tablets and thin films.
Liberation is the first step in the process by which medication enters the body and liberates the active ingredient that has been administered. The pharmaceutical drug must separate from the vehicle or the excipient that it was mixed with during manufacture. Some authors split the process of liberation into three steps: disintegration, disaggregation and dissolution. A limiting factor in the adsorption of pharmaceutical drugs is the degree to which they are ionized, as cell membranes are relatively impermeable to ionized molecules.

Furegrelate, also known as 5-(3-pyridinylmethyl)benzofurancarboxylic acid, is a chemical compound with thromboxane enzyme inhibiting properties that was originally developed by Pharmacia Corporation as a drug to treat arrhythmias, ischaemic heart disorders, and thrombosis but was discontinued. It is commercially available in the form furegrelate sodium salt.
Topical drug delivery (TDD) is a route of drug administration that allows the topical formulation to be delivered across the skin upon application, hence producing a localized effect to treat skin disorders like eczema. The formulation of topical drugs can be classified into corticosteroids, antibiotics, antiseptics, and anti-fungal. The mechanism of topical delivery includes the diffusion and metabolism of drugs in the skin. Historically, topical route was the first route of medication used to deliver drugs in humans in ancient Egyptian and Babylonian in 3000 BCE. In these ancient cities, topical medications like ointments and potions were used on the skin. The delivery of topical drugs needs to pass through multiple skin layers and undergo pharmacokinetics, hence factor like dermal diseases minimize the bioavailability of the topical drugs. The wide use of topical drugs leads to the advancement in topical drug delivery. These advancements are used to enhance the delivery of topical medications to the skin by using chemical and physical agents. For chemical agents, carriers like liposomes and nanotechnologies are used to enhance the absorption of topical drugs. On the other hand, physical agents, like micro-needles is other approach for enhancement ofabsorption. Besides using carriers, other factors such as pH, lipophilicity, and drug molecule size govern the effectiveness of topical formulation.









