
Luciferase is a generic term for the class of oxidative enzymes that produce bioluminescence, and is usually distinguished from a photoprotein. The name was first used by Raphaël Dubois who invented the words luciferin and luciferase, for the substrate and enzyme, respectively. Both words are derived from the Latin word lucifer, meaning "lightbearer", which in turn is derived from the Latin words for "light" (lux) and "to bring or carry" (ferre).

Luciferin is a generic term for the light-emitting compound found in organisms that generate bioluminescence. Luciferins typically undergo an enzyme-catalyzed reaction with molecular oxygen. The resulting transformation, which usually involves breaking off a molecular fragment, produces an excited state intermediate that emits light upon decaying to its ground state. The term may refer to molecules that are substrates for both luciferases and photoproteins.
In enzymology, a 4-hydroxyacetophenone monooxygenase (EC 1.14.13.84) is an enzyme that catalyzes the chemical reaction:
In enzymology, an alkanesulfonate monooxygenase (EC 1.14.14.5) is an enzyme that catalyzes the chemical reaction
In enzymology, an alkene monooxygenase (EC 1.14.13.69) is an enzyme that catalyzes the chemical reaction
In enzymology, a benzoyl-CoA 3-monooxygenase (EC 1.14.13.58) is an enzyme that catalyzes the chemical reaction:

In enzymology, a camphor 5-monooxygenase (EC 1.14.15.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a choline monooxygenase (EC 1.14.15.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a Latia-luciferin monooxygenase (demethylating) (EC 1.14.99.21) is an enzyme that catalyzes the chemical reaction
In enzymology, a phenylacetone monooxygenase (EC 1.14.13.92) is an enzyme that catalyzes the chemical reaction
In enzymology, a salicylate 1-monooxygenase (EC 1.14.13.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a senecionine N-oxygenase (EC 1.14.13.101) is an enzyme that catalyzes the chemical reaction
In enzymology, a thiamine oxidase (EC 1.1.3.23) is an enzyme that catalyzes the chemical reaction
In enzymology, a Cypridina-luciferin 2-monooxygenase (EC 1.13.12.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a lactate 2-monooxygenase (EC 1.13.12.4) is an enzyme that catalyzes the chemical reaction
Flavin reductase a class of enzymes. There are a variety of flavin reductases, which bind free flavins and through hydrogen bonding, catalyze the reduction of these molecules to a reduced flavin. Riboflavin, or vitamin B, and flavin mononucleotide are two of the most well known flavins in the body and are used in a variety of processes which include metabolism of fat and ketones and the reduction of methemoglobin in erythrocytes. Flavin reductases are similar and often confused for ferric reductases because of their similar catalytic mechanism and structures.
In enzymology, an FMN reductase (EC 1.5.1.29) is an enzyme that catalyzes the chemical reaction

John Woodland "Woody" Hastings, was a leader in the field of photobiology, especially bioluminescence, and was one of the founders of the field of circadian biology. He was the Paul C. Mangelsdorf Professor of Natural Sciences and Professor of Molecular and Cellular Biology at Harvard University. He published over 400 papers and co-edited three books.
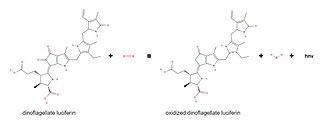
Dinoflagellate luciferase (EC 1.13.12.18, Gonyaulax luciferase) is a specific luciferase, an enzyme with systematic name dinoflagellate-luciferin:oxygen 132-oxidoreductase.

Bioluminescent bacteria are light-producing bacteria that are predominantly present in sea water, marine sediments, the surface of decomposing fish and in the gut of marine animals. While not as common, bacterial bioluminescence is also found in terrestrial and freshwater bacteria. These bacteria may be free living or in symbiosis with animals such as the Hawaiian Bobtail squid or terrestrial nematodes. The host organisms provide these bacteria a safe home and sufficient nutrition. In exchange, the hosts use the light produced by the bacteria for camouflage, prey and/or mate attraction. Bioluminescent bacteria have evolved symbiotic relationships with other organisms in which both participants benefit close to equally. Another possible reason bacteria use luminescence reaction is for quorum sensing, an ability to regulate gene expression in response to bacterial cell density.






