
A Carnot heat engine is a theoretical engine that operates on the reversible Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded upon by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857 from which the concept of entropy emerged.

In statistical mechanics, entropy is an extensive property of a thermodynamic system. It is closely related to the number Ω of microscopic configurations that are consistent with the macroscopic quantities that characterize the system. Under the assumption that each microstate is equally probable, the entropy is the natural logarithm of the number of microstates, multiplied by the Boltzmann constant kB. Formally,

Enthalpy, a property of a thermodynamic system, is equal to the system's internal energy plus the product of its pressure and volume. In a system enclosed so as to prevent matter transfer, for processes at constant pressure, the heat absorbed or released equals the change in enthalpy.

In thermodynamics and engineering, a heat engine is a system that converts heat or thermal energy—and chemical energy—to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the high temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a low temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, a lot of heat is lost to the surroundings and so cannot be converted to work.
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by , , or .
Thermal conduction is the transfer of heat by microscopic collisions of particles and movement of electrons within an organ. The microscopically colliding particles, that include molecules, atoms and electrons, transfer disorganized microscopic kinetic and potential energy, jointly known as internal energy. Conduction takes place in all phases of matter including solids, liquids, gases and waves. The rate at which energy is conducted as heat between two bodies is a function of the temperature difference between the two bodies and the properties of the conductive medium through which the heat is transferred.

The second law of thermodynamics states that the total entropy of an isolated system can never decrease over time. The total entropy of a system and its surroundings can remain constant in ideal cases where the system is in thermodynamic equilibrium, or is undergoing a (fictive) reversible process. In all processes that occur, including spontaneous processes, the total entropy of the system and its surroundings increases and the process is irreversible in the thermodynamic sense. The increase in entropy accounts for the irreversibility of natural processes, and the asymmetry between future and past.

A vacuum flask is an insulating storage vessel that greatly lengthens the time over which its contents remain hotter or cooler than the flask's surroundings. Invented by Sir James Dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. The gap between the two flasks is partially evacuated of air, creating a near-vacuum which significantly reduces heat transfer by conduction or convection.

Latent heat is thermal energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition.

The first law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic systems. The law of conservation of energy states that the total energy of an isolated system is constant; energy can be transformed from one form to another, but can be neither created nor destroyed. The first law is often formulated

Heat capacity or thermal capacity is a measurable physical quantity equal to the ratio of the heat added to an object to the resulting temperature change. The unit of heat capacity is joule per kelvin , or kilogram metre squared per kelvin second squared in the International System of Units (SI). The dimensional form is L2MT−2Θ−1. Specific heat is the amount of heat needed to raise the temperature of one kilogram of mass by 1 kelvin.

Carnot's theorem, developed in 1824 by Nicolas Léonard Sadi Carnot, also called Carnot's rule, is a principle that specifies limits on the maximum efficiency any heat engine can obtain. The efficiency of a Carnot engine depends solely on the difference between the hot and cold temperature reservoirs.

An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0. The heat transferred to the system does work, but also changes the internal energy of the system. This article uses the chemistry sign convention for work, where positive work is work done on the system. Using this convention, by the first law of thermodynamics,
The coefficient of performance or COP of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work required. Higher COPs equate to lower operating costs. The COP usually exceeds 1, especially in heat pumps, because, instead of just converting work to heat, it pumps additional heat from a heat source to where the heat is required. For complete systems, COP calculations should include energy consumption of all power consuming auxiliaries. COP is highly dependent on operating conditions, especially absolute temperature and relative temperature between sink and system, and is often graphed or averaged against expected conditions.

The Rankine cycle is a model used to predict the performance of steam turbine systems. It was also used to study the performance of reciprocating steam engines. The Rankine cycle is an idealized thermodynamic cycle of a heat engine that converts heat into mechanical work while undergoing phase change. It is an idealized cycle in which friction losses in each of the four components are neglected. The heat is supplied externally to a closed loop, which usually uses water as the working fluid. It is named after William John Macquorn Rankine, a Scottish polymath and Glasgow University professor.

In thermodynamics, the thermal efficiency is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, a steam turbine or a steam engine, a boiler, furnace, or a refrigerator for example. For a heat engine, thermal efficiency is the fraction of the energy added by heat that is converted to net work output. In the case of a refrigeration or heat pump cycle, thermal efficiency is the ratio of net heat output for heating, or removal for cooling, to energy input.

The Clausius theorem (1855) states that a system exchanging heat with external reservoirs and undergoing a cyclic process, is one that ultimately returns a system to its original state,

The Carnot cycle is a theoretical thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. It provides an upper limit on the efficiency that any classical thermodynamic engine can achieve during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference by the application of work to the system. It is not an actual thermodynamic cycle but is a theoretical construct.
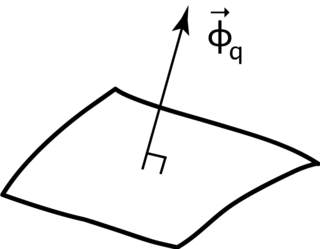
Heat flux or thermal flux, sometimes also referred to as heat flux density or heat flow rate intensity is a flow of energy per unit of area per unit of time. In SI its units are watts per square metre (W⋅m−2). It has both a direction and a magnitude, and so it is a vector quantity. To define the heat flux at a certain point in space, one takes the limiting case where the size of the surface becomes infinitesimally small.

In thermodynamics, heat is energy in transfer to or from a thermodynamic system, by mechanisms other than thermodynamic work or transfer of matter. The mechanisms include conduction, through direct contact of immobile bodies, or through a wall or barrier that is impermeable to matter; or radiation between separated bodies; or isochoric mechanical work done by the surroundings on the system of interest; or Joule heating by an electric current driven through the system of interest by an external system; or a combination of these. When there is a suitable path between two systems with different temperatures, heat transfer occurs necessarily, immediately, and spontaneously from the hotter to the colder system. Thermal conduction occurs by the stochastic (random) motion of microscopic particles. In contrast, thermodynamic work is defined by mechanisms that act macroscopically and directly on the system's whole-body state variables; for example, change of the system's volume through a piston's motion with externally measurable force; or change of the system's internal electric polarization through an externally measurable change in electric field. The definition of heat transfer does not require that the process be in any sense smooth. For example, a bolt of lightning may transfer heat to a body.






