
Botryococcus braunii is a green, pyramid-shaped planktonic microalga that is of potentially great importance in the field of biotechnology. Colonies held together by a lipid biofilm matrix can be found in temperate or tropical oligotrophic lakes and estuaries, and will bloom when in the presence of elevated levels of dissolved inorganic phosphorus. The species is notable for its ability to produce high amounts of hydrocarbons, especially oils in the form of Triterpenes, that are typically around 30–40% of their dry weight. Compared to other green alge species it has a relatively thick cell wall that is accumulated from previous cellular divisions; making extraction of cytoplasmic components rather difficult. Much of the useful hydrocarbon oil is outside of the cell.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

Prenyltransferases (PTs) are a class of enzymes that transfer allylic prenyl groups to acceptor molecules. Prenyl transferases commonly refer to isoprenyl diphosphate syntheses (IPPSs). Prenyltransferases are a functional category and include several enzyme groups that are evolutionarily independent.

Botryococcus is a genus of green algae. The cells form an irregularly shaped aggregate. Thin filaments connect the cells. The cell body is ovoid, 6 to 10 μm long, and 3 to 6 μm wide. Fossils of the genus are known since Precambrian times, and form the single largest biological contributor to crude oil, and are a major component of oil shales.
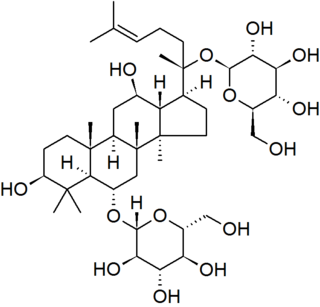
Ginsenosides or panaxosides are a class of natural product steroid glycosides and triterpene saponins. Compounds in this family are found almost exclusively in the plant genus Panax (ginseng), which has a long history of use in traditional medicine that has led to the study of pharmacological effects of ginseng compounds. As a class, ginsenosides exhibit a large variety of subtle and difficult-to-characterize biological effects when studied in isolation.
In enzymology, a GDP-L-fucose synthase (EC 1.1.1.271) is an enzyme that catalyzes the chemical reaction

[Methionine synthase] reductase, or Methionine synthase reductase, encoded by the gene MTRR, is an enzyme that is responsible for the reduction of methionine synthase inside human body. This enzyme is crucial for maintaining the one carbon metabolism, specifically the folate cycle. The enzyme employs one coenzyme, flavoprotein.
The enzyme amorpha-4,11-diene synthase (ADS) catalyzes the chemical reaction
Vetispiradiene synthase is an enzyme from Egyptian henbane that catalyzes the following chemical reaction:
In enzymology, a geranyltranstransferase is an enzyme that catalyzes the chemical reaction

Zaragozic acids are a family of natural products produced by fungi. The first characterized zaragozic acids, A, B, and C were isolated from an unidentified sterile fungal culture, Sporormiella intermedia, and L. elatius, respectively. just outside the European city Zaragoza, Spain on the Jalón river. This family of natural products possesses a unique 4,8-dioxabicyclo[3.2.1]octane core, and vary in their 1-alkyl and their 6-acyl side chains.
Phytoene synthase is a transferase enzyme involved in the biosynthesis of carotenoids. It catalyzes the conversion of geranylgeranyl pyrophosphate to phytoene. This enzyme catalyses the following chemical reaction
Botryococcene synthase (EC 1.3.1.97, SSL-3 (gene)) is an enzyme with systematic name C30 botryococcene:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
Squalene methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:squalene C-methyltransferase. This enzyme catalyses the following chemical reaction
Botryococcene C-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:botryococcene C-methyltransferase.
Presqualene diphosphate synthase is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate:(2E,6E)-farnesyl-diphosphate farnesyltransferase . This enzyme catalyses the following chemical reaction
The squalene/phytoene synthase family represents proteins that catalyze the head-to-head condensation of C15 and C20 prenyl units (i.e. farnesyl diphosphate and genranylgeranyl diphosphate). This enzymatic step constitutes part of steroid and carotenoid biosynthesis pathway. Squalene synthase EC (SQS) and Phytoene synthase EC (PSY) are two well-known examples of this protein family and share a number of functional similarities. These similarities are also reflected in their primary structure. In particular three well conserved regions are shared by SQS and PSY; they could be involved in substrate binding and/or the catalytic mechanism. SQS catalyzes the conversion of two molecules of farnesyl diphosphate (FPP) into squalene. It is the first committed step in the cholesterol biosynthetic pathway. The reaction carried out by SQS is catalyzed in two separate steps: the first is a head-to-head condensation of the two molecules of FPP to form presqualene diphosphate; this intermediate is then rearranged in a NADP-dependent reduction, to form squalene:
Syn-copalyl-diphosphate synthase is an enzyme with systematic name 9alpha-copalyl-diphosphate lyase (decyclizing). This enzyme catalyses the following chemical reaction
Halimadienyl-diphosphate synthase is an enzyme with systematic name halima-5,13-dien-15-yl-diphosphate lyase (decyclizing). This enzyme catalyses the following chemical reaction






