
Catechol-O-methyltransferase is one of several enzymes that degrade catecholamines, catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-O-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957.

l-DOPA, also known as l-3,4-dihydroxyphenylalanine and used medically as levodopa, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine.
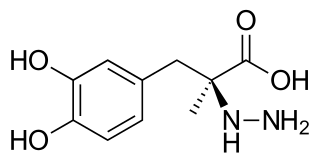
Carbidopa (Lodosyn) is a drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of administered levodopa to cross the blood–brain barrier for central nervous system effect, instead of being peripherally metabolised into substances unable to cross said barrier.

Benserazide is a peripherally acting aromatic L-amino acid decarboxylase or DOPA decarboxylase inhibitor, which is unable to cross the blood–brain barrier.
Carbidopa/levodopa, also known as levocarb and co-careldopa, is the combination of the two medications carbidopa and levodopa. It is primarily used to manage the symptoms of Parkinson's disease, but it does not slow down the disease or stop it from getting worse. It is taken by mouth. It can take two to three weeks of treatment before benefits are seen. Each dose then begins working in about ten minutes to two hours with a duration of effect of about five hours.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.
Bial is a pharmaceutical company headquartered in São Mamede do Coronado, in Trofa, Porto district, Portugal. It was founded in 1924, being among the largest companies of its kind in Portugal. Its products are sold in pharmacies in more than 58 countries in 4 continents: Europe, America, Africa and Asia.

Entacapone, sold under the brand name Comtan among others, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease. Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone.
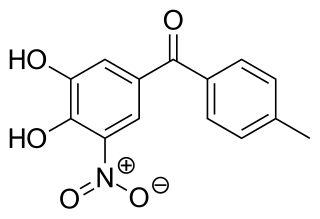
Tolcapone, sold under the brand name Tasmar, is a medication used to treat Parkinson's disease (PD). It is a selective, potent and reversible nitrocatechol-type inhibitor of the enzyme catechol-O-methyltransferase (COMT). It has demonstrated significant liver toxicity, which has led to suspension of marketing authorisations in a number of countries.
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms.

Levodopa, also known as L-DOPA and sold under many brand names, is a dopaminergic medication which is used in the treatment of Parkinson's disease and certain other conditions like dopamine-responsive dystonia and restless legs syndrome. The drug is usually used and formulated in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor like carbidopa or benserazide. Levodopa is taken by mouth, by inhalation, through an intestinal tube, or by administration into fat.
Carbidopa/levodopa/entacapone, sold under the brand name Stalevo among others, is a dopaminergic fixed-dose combination medication that contains carbidopa, levodopa, and entacapone for the treatment of Parkinson's disease.

An aromatic L-amino acid decarboxylase inhibitor (synonyms: DOPA decarboxylase inhibitor, extracerebral decarboxylase inhibitor, DDCI and AAADI) is a medication of type enzyme inhibitor which inhibits the synthesis of dopamine by the enzyme aromatic L-amino acid decarboxylase (AADC, AAAD, or DOPA decarboxylase). It is used to inhibit the decarboxylation of L-DOPA to dopamine outside the brain, i.e. in the blood. This is primarily co-administered with L-DOPA to combat Parkinson's disease. Administration can prevent common side-effects, such as nausea and vomiting, as a result of interaction with D2 receptors in the vomiting center (or cheomoreceptor trigger zone) located outside the blood–brain barrier.

Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease of mainly the central nervous system that affects both the motor and non-motor systems of the body. The symptoms usually emerge slowly, and, as the disease progresses, non-motor symptoms become more common. Usual symptoms include tremors, slowness of movement, rigidity, and difficulty with balance, collectively known as parkinsonism. Parkinson's disease dementia, falls and neuropsychiatric problems such as sleep abnormalities, psychosis, mood swings, or behavioral changes may also arise in advanced stages.
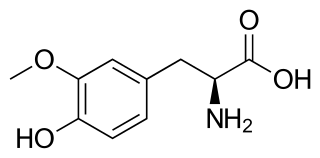
3-O-Methyldopa (3-OMD) is one of the most important metabolites of L-DOPA, a drug used in the treatment of the Parkinson's disease.
Peripherally selective drugs have their primary mechanism of action outside of the central nervous system (CNS), usually because they are excluded from the CNS by the blood–brain barrier. By being excluded from the CNS, drugs may act on the rest of the body without producing side-effects related to their effects on the brain or spinal cord. For example, most opioids cause sedation when given at a sufficiently high dose, but peripherally selective opioids can act on the rest of the body without entering the brain and are less likely to cause sedation. These peripherally selective opioids can be used as antidiarrheals, for instance loperamide (Imodium).
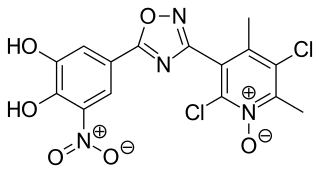
Opicapone, sold under the brand name Ongentys, is a medication which is administered together with levodopa in people with Parkinson's disease. Opicapone is a catechol-O-methyltransferase (COMT) inhibitor.

Monoamine precursors are precursors of monoamines and monoamine neurotransmitters in the body. The amino acids L-tryptophan and L-5-hydroxytryptophan are precursors of serotonin and melatonin, while the amino acids L-phenylalanine, L-tyrosine, and L-DOPA (levodopa) are precursors of dopamine, epinephrine (adrenaline), and norepinephrine (noradrenaline).
Disorders of diminished motivation (DDM) are a group of disorders involving diminished motivation and associated emotions. Many different terms have been used to refer to diminished motivation. Often however, a spectrum is defined encompassing apathy, abulia, and akinetic mutism, with apathy the least severe and akinetic mutism the most extreme.
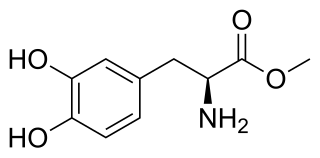
Melevodopa/carbidopa, sold under the brand name Sirio, is a combination of melevodopa, a prodrug of the dopamine precursor and hence non-selective dopamine receptor agonist levodopa (L-DOPA), and carbidopa, a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor, which is used in the treatment of Parkinson's disease in Italy. It is taken orally in the form of tablets.














