
A mineral is, broadly speaking, a solid chemical compound that occurs naturally in pure form. Minerals are most commonly associated with rocks due to the presence of minerals within rocks. These rocks may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases. Compounds that occur only in living beings are usually excluded, but some minerals are often biogenic or are organic compounds in the sense of chemistry. Moreover, living beings often synthesize inorganic minerals that also occur in rocks.

Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, and lead are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C respectively are the second-highest among all the metalloids and nonmetals, being only surpassed by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is most widely distributed in dusts, sands, planetoids, and planets as various forms of silicon dioxide (silica) or silicates. More than 90% of the Earth's crust is composed of silicate minerals, making silicon the second most abundant element in the Earth's crust after oxygen.

Amphibole is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain SiO
4 tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.
Silane is an inorganic compound with chemical formula, SiH4, making it a group 14 hydride. It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon.
Hapkeite is a mineral discovered in the Dhofar 280 meteorite found in 2000 in Oman on the Arabian peninsula. The meteorite is believed to originate from the Moon; specifically, it appears to be a fragment of lunar highland breccia. Hapkeite's composition is of silicon and iron, and it is similar to other silicon-iron minerals found on Earth. An impact on the Moon is thought to have launched the partially molten or vaporized material into orbit.

Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of the Earth's crust.
A silicide is a compound that has silicon with (usually) more electropositive elements.

Ferrosilicon is an alloy of iron and silicon with an average silicon content between 15 and 90 weight percent. It contains a high proportion of iron silicides.
Xifengite (Fe5Si3) is a rare metallic iron silicide mineral. The crystal system of xifengite is hexagonal. It has a specific gravity of 6.45 and a Mohs hardness of 5.5. It occurs as steel gray inclusions within other meteorite derived nickel iron mineral phases.
Suessite is a rare iron silicide mineral with chemical formula: Fe3Si. The mineral was named after Professor Hans E. Suess. It was discovered in 1982 during the chemical analysis of The North Haig olivine pigeonite achondrite (ureilite). It is a cream white color in reflected light, and ranges in size from 1 μm "blebs" to elongated grains that can reach up to 0.45 cm in length. This mineral belongs in the isometric crystal class. The isometric class has crystallographic axes that are all the same length and each of the three axes perpendicular to the other two. It is isotropic, has a structural type of DO3 and a crystal lattice of BiF3.

Binary compounds of silicon are binary chemical compounds containing silicon and one other chemical element. Technically the term silicide is reserved for any compounds containing silicon bonded to a more electropositive element. Binary silicon compounds can be grouped into several classes. Saltlike silicides are formed with the electropositive s-block metals. Covalent silicides and silicon compounds occur with hydrogen and the elements in groups 10 to 17.
Chromium(II) silicide (chromium disilicide) is an inorganic compound. Its chemical formula is CrSi2. It is a p-type thermoelectric semiconductor.

Nickel silicides include several intermetallic compounds of nickel and silicon. Nickel silicides are important in microelectronics as they form at junctions of nickel and silicon. Additionally thin layers of nickel silicides may have application in imparting surface resistance to nickel alloys.
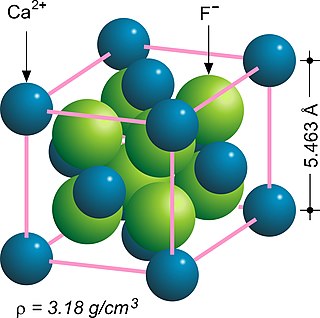
In solid state chemistry, the fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure.
Linzhiite is an iron silicide mineral with the formula FeSi2. It was discovered in the 1960s in Donetsk Oblast in Soviet Union, and named ferdisilicite, but was not approved by the International Mineralogical Association. It was later rediscovered near Lnzhi in Tibet. Linzhiite occurs together with other rare iron silicide minerals, xifengite (Fe5Si3) and naquite (FeSi).
Naquite is an iron silicide mineral with the formula FeSi. It was discovered in the 1960s in Donetsk Oblast in Soviet Union, and named fersilicite, but was not approved by the International Mineralogical Association. It was later rediscovered in Nagqu, Tibet. Naquite occurs together with other rare iron silicide minerals, xifengite (Fe5Si3) and linzhiite (FeSi2).
Iron silicide may refer to the following chemical compounds:
Iron disilicide (FeSi2) is an intermetallic compound, a silicide of iron that occurs in nature as the rare mineral linzhiite. At room temperature it forms orthorhombic crystals (β phase), which convert into a tetragonal α phase upon heating to 970 °C.

Iron monosilicide (FeSi) is an intermetallic compound, a silicide of iron that occurs in nature as the rare mineral naquite. It is a semiconductor with unusual magnetic properties at low temperatures. It has a cubic crystal lattice with no inversion center; therefore its magnetic structure is helical, with right-hand and left-handed chiralities.
Manganese monosilicide (MnSi) is an intermetallic compound, a silicide of manganese. It occurs in cosmic dust as the mineral brownleeite. MnSi has a cubic crystal lattice with no inversion center; therefore its crystal structure is helical, with right-hand and left-hand chiralities.







