Related Research Articles
Ataxia is a neurological sign consisting of lack of voluntary coordination of muscle movements that can include gait abnormality, speech changes, and abnormalities in eye movements, that indicates dysfunction of parts of the nervous system that coordinate movement, such as the cerebellum.

The cerebellum is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as it or even larger. In humans, the cerebellum plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language as well as emotional control such as regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity. Cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning in humans.
Dysarthria is a speech sound disorder resulting from neurological injury of the motor component of the motor–speech system and is characterized by poor articulation of phonemes. In other words, it is a condition in which problems effectively occur with the muscles that help produce speech, often making it very difficult to pronounce words. It is unrelated to problems with understanding language, although a person can have both. Any of the speech subsystems can be affected, leading to impairments in intelligibility, audibility, naturalness, and efficiency of vocal communication. Dysarthria that has progressed to a total loss of speech is referred to as anarthria. The term dysarthria is from Neo-Latin, dys- "dysfunctional, impaired" and arthr- "joint, vocal articulation".

The inferior olivary nucleus (ION), is a structure found in the medulla oblongata underneath the superior olivary nucleus. In vertebrates, the ION is known to coordinate signals from the spinal cord to the cerebellum to regulate motor coordination and learning. These connections have been shown to be tightly associated, as degeneration of either the cerebellum or the ION results in degeneration of the other.
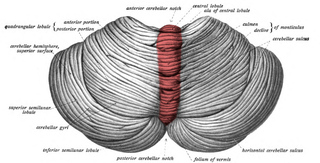
The cerebellar vermis is located in the medial, cortico-nuclear zone of the cerebellum, which is in the posterior fossa of the cranium. The primary fissure in the vermis curves ventrolaterally to the superior surface of the cerebellum, dividing it into anterior and posterior lobes. Functionally, the vermis is associated with bodily posture and locomotion. The vermis is included within the spinocerebellum and receives somatic sensory input from the head and proximal body parts via ascending spinal pathways.
Spinocerebellar ataxia (SCA) is a progressive, degenerative, genetic disease with multiple types, each of which could be considered a neurological condition in its own right. An estimated 150,000 people in the United States have a diagnosis of spinocerebellar ataxia at any given time. SCA is hereditary, progressive, degenerative, and often fatal. There is no known effective treatment or cure. SCA can affect anyone of any age. The disease is caused by either a recessive or dominant gene. In many cases people are not aware that they carry a relevant gene until they have children who begin to show signs of having the disorder.
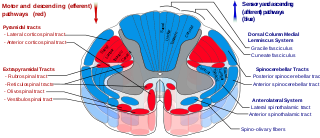
Romberg's test, Romberg's sign, or the Romberg maneuver is a test used in an exam of neurological function for balance. The exam is based on the premise that a person requires at least two of the three following senses to maintain balance while standing: proprioception ; vestibular function ; and vision.

The flocculus is a small lobe of the cerebellum at the posterior border of the middle cerebellar peduncle anterior to the biventer lobule. Like other parts of the cerebellum, the flocculus is involved in motor control. It is an essential part of the vestibulo-ocular reflex, and aids in the learning of basic motor skills in the brain.
Cerebellar ataxia is a form of ataxia originating in the cerebellum. Non-progressive congenital ataxia (NPCA) is a classical presentation of cerebral ataxias.
Intention tremor is a dyskinetic disorder characterized by a broad, coarse, and low-frequency tremor evident during deliberate and visually-guided movement. An intention tremor is usually perpendicular to the direction of movement. When experiencing an intention tremor, one often overshoots or undershoots one's target, a condition known as dysmetria. Intention tremor is the result of dysfunction of the cerebellum, particularly on the same side as the tremor in the lateral zone, which controls visually guided movements. Depending on the location of cerebellar damage, these tremors can be either unilateral or bilateral.
Uner Tan syndrome (UTS) is a syndrome that was discovered by the Turkish evolutionary biologist Üner Tan. People affected by UTS walk with a quadrupedal locomotion and often have severe learning disabilities. Tan postulated that this is an example of "reverse evolution" (atavism). The proposed syndrome was featured in the 2006 BBC2 documentary The Family That Walks On All Fours.
Benedikt syndrome, also called Benedikt's syndrome or paramedian midbrain syndrome, is a rare type of posterior circulation stroke of the brain, with a range of neurological symptoms affecting the midbrain, cerebellum and other related structures.

Dyschronometria is a condition of cerebellar dysfunction in which an individual cannot accurately estimate the amount of time that has passed. It is associated with cerebellar ataxia, when the cerebellum has been damaged and does not function to its fullest ability. Lesions to the cerebellum can cause dyssynergia, dysmetria, dysdiadochokinesia, dysarthria, and ataxia of stance and gait. Dyschronometria can result from autosomal dominant cerebellar ataxia (ADCA).

Vestibulocerebellar syndrome, also known as vestibulocerebellar ataxia, is a progressive neurological disorder that causes a variety of medical problems. Initially symptoms present as periodic attacks of abnormal eye movements but may intensify to longer-lasting motor incapacity. The disorder has been localized to the vestibulocerebellum, specifically the flocculonodular lobe. Symptoms of vestibulocerebellar syndrome may appear in early childhood but the full onset of neurological symptoms including nystagmus, ataxia, and tinnitus does not occur until early adulthood. To date, vestibulocerebellar syndrome has only been identified in three families but has affected multiple generations within them. Based on the familial pedigrees it has been characterized as an autosomal dominant disorder, although the exact genetic locus has not been identified. It has been found to be genetically distinct from other seemingly similar forms of neurological syndromes such as episodic ataxia types 1 and 2. Due to its rarity, however, little is known about specific details of the pathology or long-term treatment options. There is currently no cure for vestibulocerebellar syndrome, although some drug therapies have been effective in alleviating particular symptoms of the disorder.
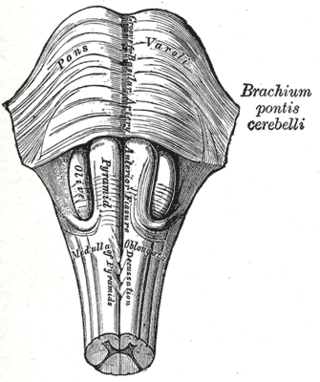
Babinski–Nageotte syndrome is an alternating brainstem syndrome. It occurs when there is damage to the dorsolateral or posterior lateral medulla oblongata, likely syphilitic in origin. Hence it is also called the alternating medulla oblongata syndrome.

Post-viral cerebellar ataxia also known as acute cerebellitis and acute cerebellar ataxia (ACA) is a disease characterized by the sudden onset of ataxia following a viral infection. The disease affects the function or structure of the cerebellum region in the brain.

Autosomal recessive cerebellar ataxia type 1 (ARCA1) is a condition characterized by progressive problems with movement. Signs and symptoms of the disorder first appear in early to mid-adulthood. People with this condition initially experience impaired speech (dysarthria), problems with coordination and balance (ataxia), or both. They may also have difficulty with movements that involve judging distance or scale (dysmetria). Other features of ARCA1 include abnormal eye movements (nystagmus) and problems following the movements of objects with their eyes. The movement problems are slowly progressive, often resulting in the need for a cane, walker, or wheelchair.
Dyssynergia is any disturbance of muscular coordination, resulting in uncoordinated and abrupt movements. This is also an aspect of ataxia. It is typical for dyssynergic patients to split a movement into several smaller movements. Types of dyssynergia include Ramsay Hunt syndrome type 1, bladder sphincter dyssynergia, and anal sphincter dyssynergia.

Gómez–López-Hernández syndrome (GLH) or cerebellotrigeminal-dermal dysplasia is a rare neurocutaneous (Phakomatosis) disorder affecting the trigeminal nerve and causing several other neural and physical abnormalities. Gómez–López-Hernández syndrome has been diagnosed in only 34 people. Cases of Gómez–López-Hernández syndrome may be under-reported as other diseases share the characteristics of cerebellar malformation shown in Gómez–López-Hernández syndrome. Gómez–López-Hernández syndrome was first characterized in 1979.

Cerebellar degeneration is a condition in which cerebellar cells, otherwise known as neurons, become damaged and progressively weaken in the cerebellum. There are two types of cerebellar degeneration; paraneoplastic cerebellar degeneration, and alcoholic or nutritional cerebellar degeneration. As the cerebellum contributes to the coordination and regulation of motor activities, as well as controlling equilibrium of the human body, any degeneration to this part of the organ can be life-threatening. Cerebellar degeneration can result in disorders in fine movement, posture, and motor learning in humans, due to a disturbance of the vestibular system. This condition may not only cause cerebellar damage on a temporary or permanent basis, but can also affect other tissues of the central nervous system, those including the cerebral cortex, spinal cord and the brainstem.
References
- ↑ "dysmetria – definition of dysmetria in the Medical dictionary – by the Free Online Medical Dictionary, Thesaurus and Encyclopedia".
- ↑ Schmahmann JD, Weilburg JB, Sherman JC (2007). "The neuropsychiatry of the cerebellum – insights from the clinic". Cerebellum. 6 (3): 254–67. doi:10.1080/14734220701490995. PMID 17786822. S2CID 14176315.
- ↑ Manto M (2009). "Mechanisms of human cerebellar dysmetria: experimental evidence and current conceptual bases". J Neuroeng Rehabil. 6: 10. doi: 10.1186/1743-0003-6-10 . PMC 2679756 . PMID 19364396.
- 1 2 3 Manto MU (2005). "The wide spectrum of spinocerebellar ataxias (SCAs)". Cerebellum. 4 (1): 2–6. doi:10.1080/14734220510007914. PMID 15895552. S2CID 5311177.
- 1 2 3 4 5 6 7 8 9 Schmahmann JD (2004). "Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome". J Neuropsychiatry Clin Neurosci. 16 (3): 367–78. doi:10.1176/jnp.16.3.367. PMID 15377747.
- ↑ Mario Manto (2010). Cerebellar Disorders: A Practical Approach to Diagnosis and Management. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-87813-5. OCLC 456170457.
- 1 2 3 Townsend J, Courchesne E, Covington J, et al. (July 1999). "Spatial attention deficits in patients with acquired or developmental cerebellar abnormality". J. Neurosci. 19 (13): 5632–43. doi: 10.1523/JNEUROSCI.19-13-05632.1999 . PMC 6782343 . PMID 10377369.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Vahdat S, Maghsoudi A, Haji Hasani M, Towhidkhah F, Gharibzadeh S, Jahed M (October 2006). "Adjustable primitive pattern generator: a novel cerebellar model for reaching movements". Neurosci. Lett. 406 (3): 232–4. doi:10.1016/j.neulet.2006.07.038. PMID 16930835. S2CID 9679947.
- 1 2 Trillenberg P, Sprenger A, Petersen D, Kömpf D, Heide W, Helmchen C (2007). "Functional dissociation of saccade and hand reaching control with bilateral lesions of the medial wall of the intraparietal sulcus: implications for optic ataxia". NeuroImage. 36 (Suppl 2): T69–76. doi:10.1016/j.neuroimage.2007.03.038. PMID 17499172. S2CID 43241167.
- ↑ Indovina I, Sanes JN (October 2001). "Combined visual attention and finger movement effects on human brain representations". Exp Brain Res. 140 (3): 265–79. doi:10.1007/s002210100796. PMID 11681302. S2CID 14364368.
- 1 2 Iwamoto Y, Yoshida K (June 2002). "Saccadic dysmetria following inactivation of the primate fastigial oculomotor region". Neurosci. Lett. 325 (3): 211–5. doi:10.1016/S0304-3940(02)00268-9. PMID 12044658. S2CID 35443728.
- ↑ Manto, M.; Godaux, E.; Jacquy, J. (January 1994). "Cerebellar hypermetria is larger when the inertial load is artificially increased". Annals of Neurology. 35 (1): 45–52. doi:10.1002/ana.410350108. ISSN 0364-5134. PMID 8285591. S2CID 19328973.
- ↑ Leggio, M.; Molinari, M. (February 2015). "Cerebellar sequencing: a trick for predicting the future". Cerebellum (London, England). 14 (1): 35–38. doi:10.1007/s12311-014-0616-x. ISSN 1473-4230. PMID 25331541. S2CID 15713873.
- 1 2 3 4 Crowdy KA, Kaur-Mann D, Cooper HL, Mansfield AG, Offord JL, Marple-Horvat DE (September 2002). "Rehearsal by eye movement improves visuomotor performance in cerebellar patients". Exp Brain Res. 146 (2): 244–7. doi:10.1007/s00221-002-1171-0. PMID 12195526. S2CID 7703727.
- 1 2 3 4 5 Hooper J, Taylor R, Pentland B, Whittle IR (April 2002). "A prospective study of thalamic deep brain stimulation for the treatment of movement disorders in multiple sclerosis". Br J Neurosurg. 16 (2): 102–9. doi:10.1080/02688690220131769. PMID 12046727. S2CID 30644098.