
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, proteins, and alcohol. The chemical energy released is available in the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a "cycle", it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.

Cellular respiration is the process by which biological fuels are oxidized in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.

Anabolism is the set of metabolic pathways that construct macromolecules like DNA or RNA from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-down aspect. Anabolism is usually synonymous with biosynthesis.
Gluconeogenesis (GNG) is a metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen (glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise.
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.

Tumor hypoxia is the situation where tumor cells have been deprived of oxygen. As a tumor grows, it rapidly outgrows its blood supply, leaving portions of the tumor with regions where the oxygen concentration is significantly lower than in healthy tissues. Hypoxic microenvironments in solid tumors are a result of available oxygen being consumed within 70 to 150 μm of tumor vasculature by rapidly proliferating tumor cells thus limiting the amount of oxygen available to diffuse further into the tumor tissue. In order to support continuous growth and proliferation in challenging hypoxic environments, cancer cells are found to alter their metabolism. Furthermore, hypoxia is known to change cell behavior and is associated with extracellular matrix remodeling and increased migratory and metastatic behavior.
The term amphibolism is used to describe a biochemical pathway that involves both catabolism and anabolism. Catabolism is a degradative phase of metabolism in which large molecules are converted into smaller and simpler molecules, which involves two types of reactions. First, hydrolysis reactions, in which catabolism is the breaking apart of molecules into smaller molecules to release energy. Examples of catabolic reactions are digestion and cellular respiration, where sugars and fats are broken down for energy. Breaking down a protein into amino acids, or a triglyceride into fatty acids, or a disaccharide into monosaccharides are all hydrolysis or catabolic reactions. Second, oxidation reactions involve the removal of hydrogens and electrons from an organic molecule. Anabolism is the biosynthesis phase of metabolism in which smaller simple precursors are converted to large and complex molecules of the cell. Anabolism has two classes of reactions. The first are dehydration synthesis reactions; these involve the joining of smaller molecules together to form larger, more complex molecules. These include the formation of carbohydrates, proteins, lipids and nucleic acids. The second are reduction reactions, in which hydrogens and electrons are added to a molecule. Whenever that is done, molecules gain energy.

Glucose 6-phosphate is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way.

3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The anion is often termed as PGA when referring to the Calvin-Benson cycle. In the Calvin-Benson cycle, 3-phosphoglycerate is typically the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO2 fixation. Thus, two equivalents of 3-phosphoglycerate are produced for each molecule of CO2 that is fixed. In glycolysis, 3-phosphoglycerate is an intermediate following the dephosphorylation (reduction) of 1,3-bisphosphoglycerate.

1,3-Bisphosphoglyceric acid (1,3-Bisphosphoglycerate or 1,3BPG) is a 3-carbon organic molecule present in most, if not all, living organisms. It primarily exists as a metabolic intermediate in both glycolysis during respiration and the Calvin cycle during photosynthesis. 1,3BPG is a transitional stage between glycerate 3-phosphate and glyceraldehyde 3-phosphate during the fixation/reduction of CO2. 1,3BPG is also a precursor to 2,3-bisphosphoglycerate which in turn is a reaction intermediate in the glycolytic pathway.
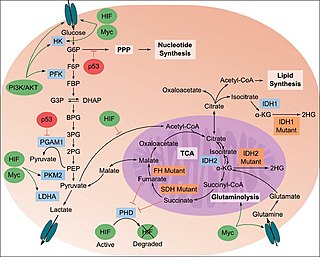
The study of the tumor metabolism, also known as tumor metabolome describes the different characteristic metabolic changes in tumor cells. The characteristic attributes of the tumor metabolome are high glycolytic enzyme activities, the expression of the pyruvate kinase isoenzyme type M2, increased channeling of glucose carbons into synthetic processes, such as nucleic acid, amino acid and phospholipid synthesis, a high rate of pyrimidine and purine de novo synthesis, a low ratio of Adenosine triphosphate and Guanosine triphosphate to Cytidine triphosphate and Uridine triphosphate, low Adenosine monophosphate levels, high glutaminolytic capacities, release of immunosuppressive substances and dependency on methionine.

Ribose 5-phosphate (R5P) is both a product and an intermediate of the pentose phosphate pathway. The last step of the oxidative reactions in the pentose phosphate pathway is the production of ribulose 5-phosphate. Depending on the body's state, ribulose 5-phosphate can reversibly isomerize to ribose 5-phosphate. Ribulose 5-phosphate can alternatively undergo a series of isomerizations as well as transaldolations and transketolations that result in the production of other pentose phosphates as well as fructose 6-phosphate and glyceraldehyde 3-phosphate.

Sucrose phosphorylase is an important enzyme in the metabolism of sucrose and regulation of other metabolic intermediates. Sucrose phosphorylase is in the class of hexosyltransferases. More specifically it has been placed in the retaining glycoside hydrolases family although it catalyzes a transglycosidation rather than hydrolysis. Sucrose phosphorylase catalyzes the conversion of sucrose to D-fructose and α-D-glucose-1-phosphate. It has been shown in multiple experiments that the enzyme catalyzes this conversion by a double displacement mechanism.
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.
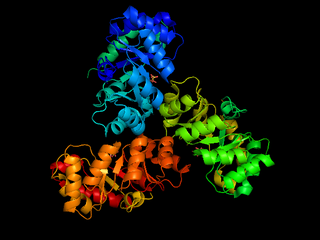
The enzyme 2-dehydro-3-deoxy-phosphogluconate aldolase, commonly known as KDPG aldolase, catalyzes the chemical reaction

Inborn errors of carbohydrate metabolism are inborn error of metabolism that affect the catabolism and anabolism of carbohydrates.
Fructolysis refers to the metabolism of fructose from dietary sources. Though the metabolism of glucose through glycolysis uses many of the same enzymes and intermediate structures as those in fructolysis, the two sugars have very different metabolic fates in human metabolism. Under one percent of ingested fructose is directly converted to plasma triglyceride. 29% - 54% of fructose is converted in liver to glucose, and about a quarter of fructose is converted to lactate. 15% - 18% is converted to glycogen. Glucose and lactate are then used normally as energy to fuel cells all over the body.
Sulfoglycolysis is a catabolic process in primary metabolism in which sulfoquinovose (6-deoxy-6-sulfonato-glucose) is metabolized to produce energy and carbon-building blocks. Sulfoglycolysis pathways occur in a wide variety of organisms, and enable key steps in the degradation of sulfoquinovosyl diacylglycerol (SQDG), a sulfolipid found in plants and cyanobacteria into sulfite and sulfate. Sulfoglycolysis converts sulfoquinovose (C6H12O8S−) into various smaller metabolizable carbon fragments such as pyruvate and dihydroxyacetone phosphate that enter central metabolism. The free energy is used to form the high-energy molecules ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Unlike glycolysis, which allows metabolism of all carbons in glucose, sulfoglycolysis pathways convert only a fraction of the carbon content of sulfoquinovose into smaller metabolizable fragments; the remainder is excreted as C3-sulfonates 2,3-dihydroxypropanesulfonate (DHPS) or sulfolactate (SL); or C2-sulfonates isethionate or sulfoacetate.














