Granite is a coarse-grained (phaneritic) intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly cools and solidifies underground. It is common in the continental crust of Earth, where it is found in igneous intrusions. These range in size from dikes only a few centimeters across to batholiths exposed over hundreds of square kilometers.

Gabbro is a phaneritic, mafic intrusive igneous rock formed from the slow cooling magma into a holocrystalline mass deep beneath the Earth's surface. Slow-cooling, coarse-grained gabbro is chemically equivalent to rapid-cooling, fine-grained basalt. Much of the Earth's oceanic crust is made of gabbro, formed at mid-ocean ridges. Gabbro is also found as plutons associated with continental volcanism. Due to its variant nature, the term gabbro may be applied loosely to a wide range of intrusive rocks, many of which are merely "gabbroic". By rough analogy, gabbro is to basalt as granite is to rhyolite.
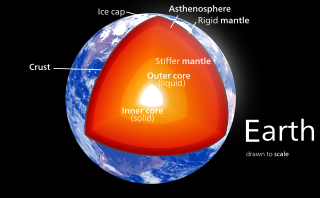
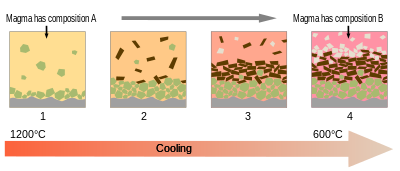
Magma is the molten or semi-molten natural material from which all igneous rocks are formed. Magma is found beneath the surface of the Earth, and evidence of magmatism has also been discovered on other terrestrial planets and some natural satellites. Besides molten rock, magma may also contain suspended crystals and gas bubbles.

A pegmatite is an igneous rock showing a very coarse texture, with large interlocking crystals usually greater in size than 1 cm (0.4 in) and sometimes greater than 1 meter (3 ft). Most pegmatites are composed of quartz, feldspar, and mica, having a similar silicic composition to granite. However, rarer intermediate composition and mafic pegmatites are known.

Plagioclase ( PLAJ-(ee)-ə-klayss, PLAYJ-, -klayz) is a series of tectosilicate (framework silicate) minerals within the feldspar group. Rather than referring to a particular mineral with a specific chemical composition, plagioclase is a continuous solid solution series, more properly known as the plagioclase feldspar series. This was first shown by the German mineralogist Johann Friedrich Christian Hessel (1796–1872) in 1826. The series ranges from albite to anorthite endmembers (with respective compositions NaAlSi3O8 to CaAl2Si2O8), where sodium and calcium atoms can substitute for each other in the mineral's crystal lattice structure. Plagioclase in hand samples is often identified by its polysynthetic crystal twinning or "record-groove" effect.

Rhyolite is the most silica-rich of volcanic rocks. It is generally glassy or fine-grained (aphanitic) in texture, but may be porphyritic, containing larger mineral crystals (phenocrysts) in an otherwise fine-grained groundmass. The mineral assemblage is predominantly quartz, sanidine, and plagioclase. It is the extrusive equivalent of granite.

Metamorphism is the transformation of existing rock to rock with a different mineral composition or texture. Metamorphism takes place at temperatures in excess of 150 °C (300 °F), and often also at elevated pressure or in the presence of chemically active fluids, but the rock remains mostly solid during the transformation. Metamorphism is distinct from weathering or diagenesis, which are changes that take place at or just beneath Earth's surface.

Andesite is a volcanic rock of intermediate composition. In a general sense, it is the intermediate type between silica-poor basalt and silica-rich rhyolite. It is fine-grained (aphanitic) to porphyritic in texture, and is composed predominantly of sodium-rich plagioclase plus pyroxene or hornblende.

Petrology is the branch of geology that studies rocks, their mineralogy, composition, texture, structure and the conditions under which they form. Petrology has three subdivisions: igneous, metamorphic, and sedimentary petrology. Igneous and metamorphic petrology are commonly taught together because both make heavy use of chemistry, chemical methods, and phase diagrams. Sedimentary petrology is commonly taught together with stratigraphy because it deals with the processes that form sedimentary rock. Modern sedimentary petrology is making increasing use of chemistry.

Peridotite ( PERR-ih-doh-tyte, pə-RID-ə-) is a dense, coarse-grained igneous rock consisting mostly of the silicate minerals olivine and pyroxene. Peridotite is ultramafic, as the rock contains less than 45% silica. It is high in magnesium (Mg2+), reflecting the high proportions of magnesium-rich olivine, with appreciable iron. Peridotite is derived from Earth's mantle, either as solid blocks and fragments, or as crystals accumulated from magmas that formed in the mantle. The compositions of peridotites from these layered igneous complexes vary widely, reflecting the relative proportions of pyroxenes, chromite, plagioclase, and amphibole.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Intrusive rock is formed when magma penetrates existing rock, crystallizes, and solidifies underground to form intrusions, such as batholiths, dikes, sills, laccoliths, and volcanic necks.

Sanidine is the high temperature form of potassium feldspar with a general formula K(AlSi3O8). Sanidine is found most typically in felsic volcanic rocks such as obsidian, rhyolite and trachyte. Sanidine crystallizes in the monoclinic crystal system. Orthoclase is a monoclinic polymorph stable at lower temperatures. At yet lower temperatures, microcline, a triclinic polymorph of potassium feldspar, is stable.

Cumulate rocks are igneous rocks formed by the accumulation of crystals from a magma either by settling or floating. Cumulate rocks are named according to their texture; cumulate texture is diagnostic of the conditions of formation of this group of igneous rocks. Cumulates can be deposited on top of other older cumulates of different composition and colour, typically giving the cumulate rock a layered or banded appearance.
In geology, igneous differentiation, or magmatic differentiation, is an umbrella term for the various processes by which magmas undergo bulk chemical change during the partial melting process, cooling, emplacement, or eruption. The sequence of magmas produced by igneous differentiation is known as a magma series.
The tholeiitic magma series is one of two main magma series in subalkaline igneous rocks, the other being the calc-alkaline series. A magma series is a chemically distinct range of magma compositions that describes the evolution of a mafic magma into a more evolved, silica rich end member. Rock types of the tholeiitic magma series include tholeiitic basalt, ferro-basalt, tholeiitic basaltic andesite, tholeiitic andesite, dacite and rhyolite. The variety of basalt in the series was originally called tholeiite but the International Union of Geological Sciences recommends that tholeiitic basalt be used in preference to that term.
The calc-alkaline magma series is one of two main subdivisions of the subalkaline magma series, the other subalkaline magma series being the tholeiitic series. A magma series is a series of compositions that describes the evolution of a mafic magma, which is high in magnesium and iron and produces basalt or gabbro, as it fractionally crystallizes to become a felsic magma, which is low in magnesium and iron and produces rhyolite or granite. Calc-alkaline rocks are rich in alkaline earths and alkali metals and make up a major part of the crust of the continents.

In geology, an igneous intrusion is a body of intrusive igneous rock that forms by crystallization of magma slowly cooling below the surface of the Earth. Intrusions have a wide variety of forms and compositions, illustrated by examples like the Palisades Sill of New York and New Jersey; the Henry Mountains of Utah; the Bushveld Igneous Complex of South Africa; Shiprock in New Mexico; the Ardnamurchan intrusion in Scotland; and the Sierra Nevada Batholith of California.
Igneous rock, or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rocks are formed through the cooling and solidification of magma or lava.

A crystal mush is magma that contains a significant amount of crystals suspended in the liquid phase (melt). As the crystal fraction makes up less than half of the volume, there is no rigid large-scale three-dimensional network as in solids. As such, their rheological behavior mirrors that of absolute liquids.