Related Research Articles

Bacillus Calmette–Guérin (BCG) vaccine is a vaccine primarily used against tuberculosis (TB). It is named after its inventors Albert Calmette and Camille Guérin. In countries where tuberculosis or leprosy is common, one dose is recommended in healthy babies as soon after birth as possible. In areas where tuberculosis is not common, only children at high risk are typically immunized, while suspected cases of tuberculosis are individually tested for and treated. Adults who do not have tuberculosis and have not been previously immunized, but are frequently exposed, may be immunized, as well. BCG also has some effectiveness against Buruli ulcer infection and other nontuberculous mycobacterial infections. Additionally, it is sometimes used as part of the treatment of bladder cancer.
In genomics, DNA–DNA hybridization is a molecular biology technique that measures the degree of genetic similarity between DNA sequences. It is used to determine the genetic distance between two organisms and has been used extensively in phylogeny and taxonomy.
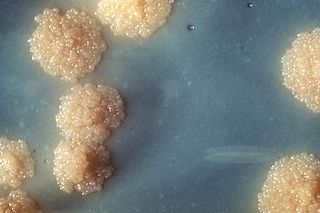
Mycobacterium tuberculosis, also known as Koch's bacillus, is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface primarily due to the presence of mycolic acid. This coating makes the cells impervious to Gram staining, and as a result, M. tuberculosis can appear weakly Gram-positive. Acid-fast stains such as Ziehl–Neelsen, or fluorescent stains such as auramine are used instead to identify M. tuberculosis with a microscope. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, it infects the lungs. The most frequently used diagnostic methods for tuberculosis are the tuberculin skin test, acid-fast stain, culture, and polymerase chain reaction.

Mycobacterium is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis and leprosy in humans. The Greek prefix myco- means 'fungus', alluding to this genus' mold-like colony surfaces. Since this genus has cell walls with a waxy lipid-rich outer layer that contains high concentrations of mycolic acid, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types.
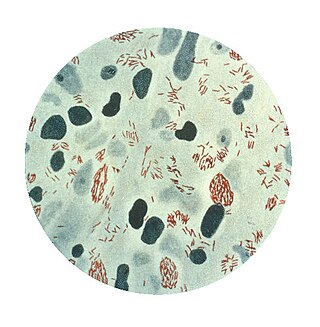
Mycobacterium leprae is one of the two species of bacteria that cause Hansen's disease (leprosy), a chronic but curable infectious disease that damages the peripheral nerves and targets the skin, eyes, nose, and muscles.

Mycobacterium smegmatis is an acid-fast bacterial species in the phylum Actinomycetota and the genus Mycobacterium. It is 3.0 to 5.0 μm long with a bacillus shape and can be stained by Ziehl–Neelsen method and the auramine-rhodamine fluorescent method. It was first reported in November 1884 by Lustgarten, who found a bacillus with the staining appearance of tubercle bacilli in syphilitic chancres. Subsequent to this, Alvarez and Tavel found organisms similar to that described by Lustgarten also in normal genital secretions (smegma). This organism was later named M. smegmatis.
The Ames strain is one of 89 known strains of the anthrax bacterium. It was isolated from a diseased 14-month-old Beefmaster heifer that died in Sarita, Texas in 1981. The strain was isolated at the Texas Veterinary Medical Diagnostic Laboratory and a sample was sent to the United States Army Medical Research Institute of Infectious Diseases (USAMRIID). Researchers at USAMRIID mistakenly believed the strain came from Ames, Iowa because the return address on the package was the USDA's National Veterinary Services Laboratories in Ames and mislabeled the specimen.
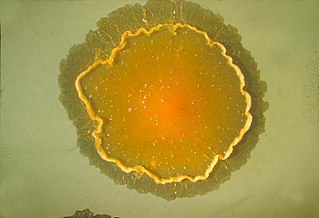
Mycobacterium marinum is a slow growing fresh and saltwater mycobacterium (SGM) belonging to the genus Mycobacterium and the phylum Actinobacteria. It was formerly known as Mycobacterium balnei. The strain marinum was first identified by Joseph D. Aronson in 1926 and it is observed as a pathogenic mycobacterium causing tuberculosis-like infections in fish (mycobacteriosis) and skin lesions in humans. The bacteria grows optimal at a temperature around 30 °C.
Lipoarabinomannan, also called LAM, is a glycolipid, and a virulence factor associated with Mycobacterium tuberculosis, the bacteria responsible for tuberculosis. Its primary function is to inactivate macrophages and scavenge oxidative radicals.

Mycobacteroides abscessus is a species of rapidly growing, multidrug-resistant, nontuberculous mycobacteria (NTM) that is a common soil and water contaminant. Although M. abscessus most commonly causes chronic lung infection and skin and soft tissue infection (SSTI), it can also cause infection in almost all human organs, mostly in patients with suppressed immune systems. Amongst NTM species responsible for disease, infection caused by M. abscessus complex are more difficult to treat due to antimicrobial drug resistance.
Mycobacteroides chelonae is a species of bacteria from the phylum Actinomycetota belonging to the genus Mycobacteroides. Mycobacteroides chelonae is a rapidly growing mycobacterium that is found all throughout the environment, including sewage and tap water. It can occasionally cause opportunistic infections of humans. It is grouped in Runyon group IV.
ESAT-6 or early secreted antigenic target 6 kDa, is produced by Mycobacterium tuberculosis, it is a secretory protein and potent T cell antigen. It is used in tuberculosis diagnosis by the whole blood interferon γ test QuantiFERON-TB Gold, in conjunction with CFP-10.

CFP-10 within bacterial proteins is a protein that is encoded by the esxB gene.
Listeriolysin O (LLO) is a hemolysin produced by the bacterium Listeria monocytogenes, the pathogen responsible for causing listeriosis. The toxin may be considered a virulence factor, since it is crucial for the virulence of L. monocytogenes.

Rhodococcus equi is a Gram-positive coccobacillus bacterium. The organism is commonly found in dry and dusty soil and can be important for diseases of domesticated animals. The frequency of infection can reach near 60%. R. equi is an important pathogen causing pneumonia in foals. Since 2008, R. equi has been known to infect wild boar and domestic pigs. R. equi can infect immunocompromised people, such as HIV-AIDS patients or organ transplant recipients. Rhodococcus equi infection in these populations resemble the clinical and pathological signs of advanced pulmonary tuberculosis. This organism is a facultative intracellular mycobacterial pathogen.

Isocitrate lyase, or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.

Cord factor, or trehalose dimycolate (TDM), is a glycolipid molecule found in the cell wall of Mycobacterium tuberculosis and similar species. It is the primary lipid found on the exterior of M. tuberculosis cells. Cord factor influences the arrangement of M. tuberculosis cells into long and slender formations, giving its name. Cord factor is virulent towards mammalian cells and critical for survival of M. tuberculosis in hosts, but not outside of hosts. Cord factor has been observed to influence immune responses, induce the formation of granulomas, and inhibit tumor growth. The antimycobacterial drug SQ109 is thought to inhibit TDM production levels and in this way disrupts its cell wall assembly.

Bacillus anthracis is a gram-positive and rod-shaped bacterium that causes anthrax, a deadly disease to livestock and, occasionally, to humans. It is the only permanent (obligate) pathogen within the genus Bacillus. Its infection is a type of zoonosis, as it is transmitted from animals to humans. It was discovered by a German physician Robert Koch in 1876, and became the first bacterium to be experimentally shown as a pathogen. The discovery was also the first scientific evidence for the germ theory of diseases.
The Mycobacterium tuberculosis complex is a genetically related group of Mycobacterium species that can cause tuberculosis in humans or other animals.
Ashwani Kumar is an Indian microbiologist and the Senior Principal Scientist at the Institute of Microbial Technology (ImTech). He is known for his studies on Mycobacterium tuberculosis, the causative agent of tuberculosis. His laboratory focuses on understanding the reasons for drug tolerance observed in humans. His laboratory hypothesizes that tuberculosis is a biofilm infection, so its treatment needs the administration of multiple drugs for at least six months. The Department of Science and Technology has awarded him Swarnajayanti Fellowship for 2016–2017. Department of Biotechnology has awarded him the National Bioscience Prize (2017-18). He was also selected for DBT/Wellcome Trust India Alliance Senior Fellowship. He was elected as a Fellow of the National Academy of Sciences, India, in 2022. For his contributions in tuberculosis pathogenesis, he was awarded with Shanti Swarup Bhatnagar Prize for Science and Technology 2022. He is considered as one of India's Leading Scientist in the field of Tuberculosis and his lab is doing some of the best research in India.
References
- ↑ Camus, Jean-Christophe; Médigue, Claudine; Cole, Stewart T.; Pryor, Melinda J. (2002-10-01). "Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv". Microbiology. 148 (10): 2967–2973. doi: 10.1099/00221287-148-10-2967 . PMID 12368430.
- 1 2 KUBICA, G. P.; KIM, T. H.; DUNBAR, F. P. (1972-04-01). "Designation of Strain H37Rv as the Neotype of Mycobacterium tuberculosis". International Journal of Systematic Bacteriology. 22 (2): 99–106. doi: 10.1099/00207713-22-2-99 .
- 1 2 Steenken, W.; Oatway, W. H.; Petroff, S. A. (1934-09-30). "Biological Studies of the Tubercle Bacillus: III. Dissociation and Pathogenicity of the R and S Variants of the Human Tubercle Bacillus (H37)". The Journal of Experimental Medicine. 60 (4): 515–540. doi:10.1084/jem.60.4.515. ISSN 0022-1007. PMC 2132400 . PMID 19870319.
- 1 2 Steenken, W. (1935). "Lysis of Tubercle Bacilli in Vitro". Experimental Biology and Medicine. 33 (2): 253–255. doi:10.3181/00379727-33-8330p. S2CID 87337106.
- ↑ Cole, S. T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S. V.; Eiglmeier, K.; Gas, S. (1998-06-11). "Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence". Nature. 393 (6685): 537–544. Bibcode:1998Natur.393..537C. doi: 10.1038/31159 . ISSN 0028-0836. PMID 9634230.
- ↑ Bouwman, Abigail S.; Kennedy, Sandra L.; Müller, Romy; Stephens, Richard H.; Holst, Malin; Caffell, Anwen C.; Roberts, Charlotte A.; Brown, Terence A. (2012-11-06). "Genotype of a historic strain of Mycobacterium tuberculosis". Proceedings of the National Academy of Sciences. 109 (45): 18511–18516. Bibcode:2012PNAS..10918511B. doi: 10.1073/pnas.1209444109 . ISSN 0027-8424. PMC 3494915 . PMID 23091009.
- ↑ Shammari, Basim Al; Shiomi, Takayuki; Tezera, Liku; Bielecka, Magdalena K.; Workman, Victoria; Sathyamoorthy, Tarangini; Mauri, Francesco; Jayasinghe, Suwan N.; Robertson, Brian D. (2015-08-01). "The Extracellular Matrix Regulates Granuloma Necrosis in Tuberculosis". Journal of Infectious Diseases. 212 (3): 463–473. doi:10.1093/infdis/jiv076. ISSN 0022-1899. PMC 4539912 . PMID 25676469.
- 1 2 3 Ioerger, Thomas R.; Feng, Yicheng; Ganesula, Krishna; Chen, Xiaohua; Dobos, Karen M.; Fortune, Sarah; Jacobs, William R.; Mizrahi, Valerie; Parish, Tanya (2010-07-15). "Variation among Genome Sequences of H37Rv Strains of Mycobacterium tuberculosis from Multiple Laboratories". Journal of Bacteriology. 192 (14): 3645–3653. doi:10.1128/JB.00166-10. ISSN 0021-9193. PMC 2897344 . PMID 20472797.