
Piper, the pepper plants or pepper vines, is an economically and ecologically important genus in the family Piperaceae.

Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, typical of amines. The name comes from the genus name Piper, which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins.

Black pepper is a flowering vine in the family Piperaceae, cultivated for its fruit, which is usually dried and used as a spice and seasoning. The fruit is a drupe (stonefruit) which is about 5 mm (0.20 in) in diameter, dark red, and contains a stone which encloses a single pepper seed. Peppercorns and the ground pepper derived from them may be described simply as pepper, or more precisely as black pepper, green pepper, or white pepper.
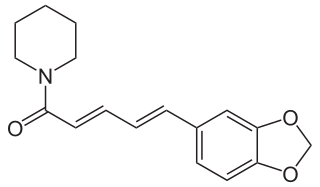
Piperine, possibly along with its isomer chavicine, is the compound responsible for the pungency of black pepper and long pepper. It has been used in some forms of traditional medicine.

Prazepam is a benzodiazepine derivative drug developed by Warner-Lambert in the 1960s. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Prazepam is a prodrug for desmethyldiazepam which is responsible for the therapeutic effects of prazepam.
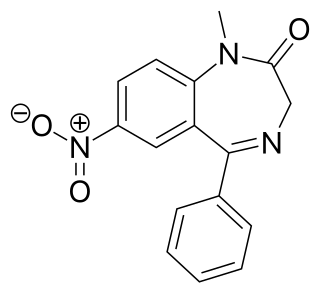
Nimetazepam is an intermediate-acting hypnotic drug which is a benzodiazepine derivative. It was first synthesized by a team at Hoffmann-La Roche in 1964. It possesses powerful hypnotic, anxiolytic, sedative, and skeletal muscle relaxant properties. Nimetazepam is also a particularly potent anticonvulsant. It is marketed in 5 mg tablets known as Erimin, which is the brand name manufactured and marketed by the large Japanese corporation Sumitomo. Japan is the sole manufacturer of nimetazepam in the world. Outside of Japan, Erimin is available in much of East and Southeast Asia and was widely prescribed for the short-term treatment of severe insomnia in patients who have difficulty falling asleep or maintaining sleep. Sumitomo has ceased manufacturing Erimin since November 2015. It is still available as a generic drug or as Lavol.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, PD-116,948) is a drug which acts as a potent and selective antagonist for the adenosine A1 receptor. It has high selectivity for A1 over other adenosine receptor subtypes, but as with other xanthine derivatives DPCPX also acts as a phosphodiesterase inhibitor, and is almost as potent as rolipram at inhibiting PDE4. It has been used to study the function of the adenosine A1 receptor in animals, which has been found to be involved in several important functions such as regulation of breathing and activity in various regions of the brain, and DPCPX has also been shown to produce behavioural effects such as increasing the hallucinogen-appropriate responding produced by the 5-HT2A agonist DOI, and the dopamine release induced by MDMA, as well as having interactions with a range of anticonvulsant drugs.

CL-218,872 is a sedative and hypnotic drug used in scientific research. It has similar effects to sedative-hypnotic benzodiazepine drugs such as triazolam, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic.

Y-23684 is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
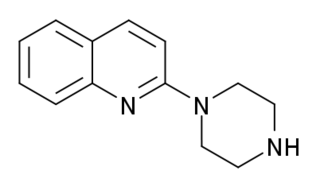
Quipazine is a serotonergic drug of the piperazine group which is used in scientific research. It was originally intended as an antidepressant but never developed for medical use.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a substituted amphetamine and monoamine releaser similar to MDMA, but with substantially higher neurotoxicity, thought to be due to the unrestrained release of both serotonin and dopamine by a metabolite. It is used as a neurotoxin by neurobiologists to selectively kill serotonergic neurons for research purposes, in the same way that 6-hydroxydopamine is used to kill dopaminergic neurons.

Tolufazepam is a drug that is a benzodiazepine derivative. Studies have shown tolufazepam to have anticonvulsant and anxiolytic activity in animal subjects, including convulsions elicited by pentylenetetrazol.
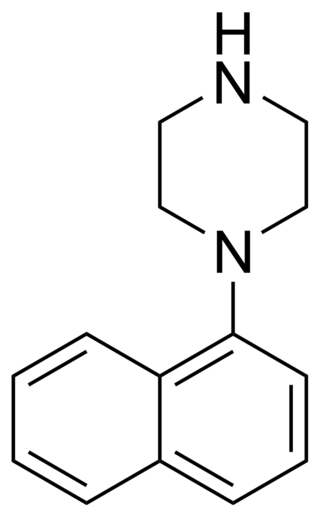
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.

Benzobarbital (Benzonal) is a barbiturate derivative. It has anticonvulsant effects and has been used for the treatment of epilepsy.

CP-1414S is an experimental drug first made by a team in Germany. It is a benzodiazepine derivative. CP-1414S is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring, and so is most closely related to other 1,5-benzodiazepines such as clobazam.

Bioenhancers or biopotentiators or bioavailability enhancers is a new chapter in medical science first scientifically established in 1979 after the discovery of world's first bioenhancer piperine. It is a pocket friendly drug technology which reduces the destruction, wastage and elimination of several orally administered drugs inside the body.

para-Chloromethamphetamine is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA). It has been found to decrease serotonin in rats. Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.

Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory. Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies, as does its 7-OH derivative.

Sergolexole (developmental code name LY-281,067) is an ergoline derivative which acts as a selective antagonist of the serotonin 5-HT2 receptors. It has been used for various research applications, but was never developed for medical use.




















