 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
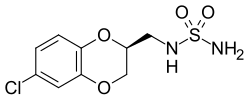
| Formula | C9H11ClN2O4S |
| Molar mass | 278.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
JNJ-26489112 is an anticonvulsant drug being developed by Johnson & Johnson for the treatment of epilepsy. [1] [2] [3] JNJ-26489112 was designed as a successor to topiramate. [4] It is expected to have fewer side effects than topiramate because it lacks activity against carbonic anhydrase. [4]
Contents
JNJ-26489112 was studied as a treatment for major depressive disorder. [5] This clinical trial was terminated in 2013 due to a "sponsor portfolio decision", and no new development of JNJ-26489112 has been reported.
Its mechanism of action is unknown. [6]