
In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

In organic chemistry, hydrocarbons are divided into two classes: aromatic compounds and aliphatic compounds. Aliphatic compounds can be saturated like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic.

In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

Carbon is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

In chemistry, many authors consider an organic compound to be any chemical compound that contains carbon–hydrogen or carbon–carbon bonds; however, some authors consider an organic compound to be any chemical compound that contains carbon. The definition of "organic" versus "inorganic", and whether some other carbon-containing compounds are organic or inorganic vary from author to author, and are topics of debate. For example, carbon-containing compounds such as alkanes and its derivatives are considered organic, but many others are considered inorganic, such as halides of carbon without carbon-hydrogen and carbon-carbon bonds, and certain compounds of carbon with nitrogen and oxygen.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.

In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds—that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
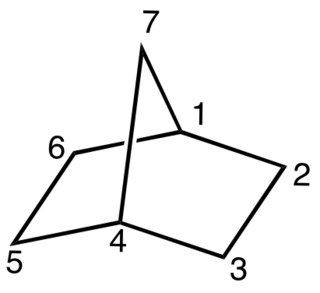
A bicyclic molecule is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic, or heterocyclic, like DABCO. Moreover, the two rings can both be aliphatic, or can be aromatic, or a combination of aliphatic and aromatic.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
In organic chemistry, the carbon number of a compound is the number of carbon atoms in each molecule. The properties of hydrocarbons can be correlated with the carbon number, although the carbon number alone does not give an indication of the saturation of the organic compound. When describing a particular molecule, the "carbon number" is also the ordinal position of a particular carbon atom in a chain.
This is an index of lists of molecules. Millions of molecules have existed in the universe since before the formation of Earth. Three of them, carbon dioxide, water and oxygen were necessary for the growth of life. Although humanity had always been surrounded by these substances, it has not always known what they were composed of.