
Mouthwash, mouth rinse, oral rinse, or mouth bath is a liquid which is held in the mouth passively or swirled around the mouth by contraction of the perioral muscles and/or movement of the head, and may be gargled, where the head is tilted back and the liquid bubbled at the back of the mouth.

Rubbing alcohol is either an isopropyl alcohol or an ethanol-based liquid, with isopropyl alcohol products being the most widely available. The comparable British Pharmacopoeia (BP) is surgical spirit. Rubbing alcohol is denatured and undrinkable even if it is ethanol-based, due to the bitterants added.

Menthol is an organic compound, specifically a monoterpenoid, that occurs naturally in the oils of several plants in the mint family, such as corn mint and peppermint. It is a white or clear waxy crystalline substance that is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1R,2S,5R) configuration.

Bad breath, also known as halitosis, is a symptom in which a noticeably unpleasant breath odour is present. It can result in anxiety among those affected. It is also associated with depression and symptoms of obsessive compulsive disorder.
Crest is an American brand of toothpaste and other oral hygiene products made by American multinational Procter & Gamble (P&G) and sold worldwide. In many countries in Europe, such as Germany, Bulgaria, Serbia, Ukraine, Belarus, Russia, Poland, Hungary, Latvia, Romania, Estonia and Lithuania, it is sold as Blend-A-Med, the name of an established German toothpaste acquired by P&G in 1987. In France, Spain, Italy, Israel, Sweden, Finland, Colombia, Belgium, the Netherlands, Brazil, the United Kingdom, the Republic of Ireland, Australia, Nigeria, Greece, Uruguay and Argentina, P&G markets similar toothpaste formulations under the Oral-B brand.

Methyl salicylate (oil of wintergreen or wintergreen oil) is an organic compound with the formula C8H8O3. It is the methyl ester of salicylic acid. It is a colorless, viscous liquid with a sweet, fruity odor reminiscent of root beer (in which it is used as a flavoring), but often associatively called "minty", as it is an ingredient in mint candies. It is produced by many species of plants, particularly wintergreens. It is also produced synthetically, used as a fragrance and as a flavoring agent.

Chlorhexidine is a disinfectant and antiseptic with the molecular formula C22H30Cl2N10, which is used for skin disinfection before surgery and to disinfect surgical instruments. It is also used for cleaning wounds, preventing dental plaque, treating yeast infections of the mouth, and to keep urinary catheters from blocking. It is used as a liquid or a powder. It is commonly used in salt form, either the gluconate or the acetate.

Liniment, also called embrocation and heat rub, is a medicated topical preparation for application to the skin. Some liniments have a viscosity similar to that of water; others are lotion or balm; still, others are in transdermal patches, soft solid sticks, and sprays. Liniment usually is rubbed into the skin, which the active ingredients penetrate.
Breath spray is a product sprayed into the mouth for the purpose of temporarily eliminating or at least covering up bad breath. The masking effect is short-term and reported to last for 4-6 hours. Breath sprays are occasionally advertised as being for smokers or those who dip tobacco, and occasionally to cover up the smell of cigarette/cigar smoking. Common flavours include cinnamon, spearmint and peppermint, as well as company-specific flavors, such as "Ice Mint", "Cool Mint" or "Supermint".

Wintergreen is a group of aromatic plants. The term wintergreen once commonly referred to plants that remain green throughout the winter. The term evergreen is now more commonly used for this characteristic.

Hexetidine is an anti-bacterial and anti-fungal agent commonly used in both veterinary and human medicine. It is a local anesthetic, astringent and deodorant and has antiplaque effects.

Eucalyptol is a monoterpenoid colorless liquid, and a bicyclic ether. It has a fresh camphor-like odor and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up about 70–90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.

Certs was a brand of breath mint that was noted for the frequent use of "two mints in one" in its marketing. The original "classic mints" were disc-shaped without a hole and sold in roll packaging similar to Life Savers and Polo. Certs was one of the first mints to be nationally marketed in the United States and has been a fixture at American drug stores and convenience stores since its debut on the market in 1956. It was discontinued in 2018, possibly due to its containing partially hydrogenated cottonseed oil, an ingredient which has not been allowed in food sold in the United States since then.
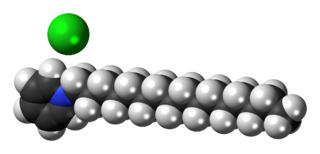
Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium compound used in some types of mouthwashes, toothpastes, lozenges, throat sprays, breath sprays, and nasal sprays. It is an antiseptic that kills bacteria and other microorganisms. It has been shown to be effective in preventing dental plaque and reducing gingivitis. It has also been used as an ingredient in certain pesticides. Though one study seems to indicate cetylpyridinium chloride does not cause brown tooth stains, at least one mouthwash containing CPC as an active ingredient bears the warning label "In some cases, antimicrobial rinses may cause surface staining to teeth," following a failed class-action lawsuit brought by customers whose teeth were stained.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.

Euthymol is a brand of antiseptic, fluoride-free toothpaste distributed by LG H&H UK that is characterised by its bright pink colour and medicinal taste. It is also notable for its packaging, which is old fashioned, having merely a pattern and the product name.

Oral hygiene is the practice of keeping one's oral cavity clean and free of disease and other problems by regular brushing of the teeth and adopting good hygiene habits. It is important that oral hygiene be carried out on a regular basis to enable prevention of dental disease and bad breath. The most common types of dental disease are tooth decay and gum diseases, including gingivitis, and periodontitis.

Dentyl Dual Action, previously known as Dentyl Active, and originally as Dentyl pH, is a brand of mouthwash, an oral hygiene product designed to reduce the presence of bacteria responsible for tooth decay, gingivitis and halitosis. Traditional mouthwash formulations typically use alcohol or other antimicrobial ingredients to kill bacteria.

Gingivitis is a non-destructive disease that causes inflammation of the gums; ulitis is an alternative term. The most common form of gingivitis, and the most common form of periodontal disease overall, is in response to bacterial biofilms that are attached to tooth surfaces, termed plaque-induced gingivitis. Most forms of gingivitis are plaque-induced.
Warner–Lambert was an American pharmaceutical company.


















