Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.
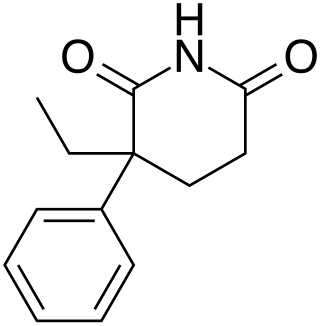
Glutethimide is a hypnotic sedative that was introduced by Ciba in 1954 as a safe alternative to barbiturates to treat insomnia. Before long, however, it had become clear that glutethimide was just as likely to cause addiction and caused similar withdrawal symptoms. Doriden was the brand-name version. Current production levels in the United States point to its use only in small-scale research. Manufacturing of the drug was discontinued in the US in 1993 and discontinued in several eastern European countries in 2006.

Amobarbital is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. It was first synthesized in Germany in 1923. It is considered a short to intermediate acting barbiturate.

Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type. It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries. In the developed world, it is commonly used to treat seizures in young children, while other medications are generally used in older children and adults. It is also used for veterinary purposes.
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active. The compound was first synthesised by Adolf von Baeyer.
Pentobarbital (US) or pentobarbitone is a short-acting barbiturate typically used as a sedative, a preanesthetic, and to control convulsions in emergencies. It can also be used for short-term treatment of insomnia but has been largely replaced by the benzodiazepine family of drugs.
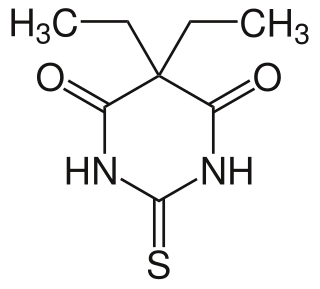
Thiobarbital is a drug which is a barbiturate derivative. It is the thiobarbiturate analogue of barbital.

Metharbital was patented in 1905 by Emil Fischer working for Merck. It was marketed as Gemonil by Abbott Laboratories. It is a barbiturate anticonvulsant, used in the treatment of epilepsy. It has similar properties to phenobarbital.
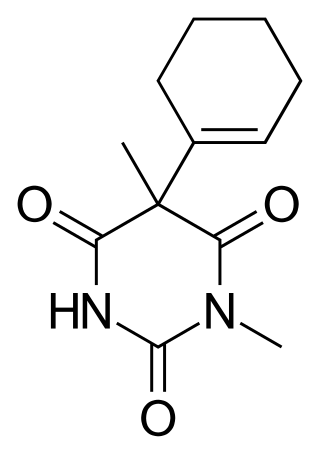
Hexobarbital or hexobarbitone, sold both in acid and sodium salt forms as Citopan, Evipan, and Tobinal, is a barbiturate derivative having hypnotic and sedative effects. It was used in the 1940s and 1950s as an agent for inducing anesthesia for surgery, as well as a rapid-acting, short-lasting hypnotic for general use, and has a relatively fast onset of effects and short duration of action. Modern barbiturates have largely supplanted the use of hexobarbital as an anesthetic, as they allow for better control of the depth of anesthesia. Hexobarbital is still used in some scientific research.

Cyclopentobarbital sodium is a barbiturate derivative invented in the 1940s. It has sedative and anticonvulsant properties, and was used primarily as an anaesthetic in veterinary medicine. Cyclopal is considered similar in effects to phenobarbital but lasts almost three times as long, and is considered a long-acting barbiturate with a fairly slow onset of action.

Hexethal (Ortal) is a barbiturate derivative invented in the 1940s. It has sedative, anxiolytic, muscle relaxant, and anticonvulsant properties, and was used primarily as an anaesthetic in veterinary medicine.
Propallylonal is a barbiturate derivative invented in the 1920s. It has sedative, hypnotic and anticonvulsant properties, and is still rarely prescribed as a sleeping medication in some Eastern-European countries.

Reposal is a barbiturate derivative invented in the 1960s in Denmark. It has sedative, hypnotic and anticonvulsant properties, and was used primarily for the treatment of insomnia.
Barbiturase is a zinc-containing amidohydrolase. Its systemic name is barbiturate amidohydrolase (3-oxo-3-ureidopropanoate-forming). Barbiturase acts as a catalyst in the second step of oxidative pyrimidine degradation, promoting the ring-opening hydrolysis of barbituric acid to ureidomalonic acid. Although grouped into the naturally existing amidohydrolases, it demonstrates more homology with cyanuric acid amidohydrolase. Therefore, it has been proposed that barbiturase, along with cyanuric acid, should be grouped into a new family. KEGG

Crotylbarbital, also known as crotarbital, is a barbiturate derivative developed by Eli Lilly in the 1930s. It has sedative and hypnotic effects, and was used for the treatment of insomnia until it was replaced by newer alternative drugs with fewer side effects and lower risk of overdose.

Benzylbutylbarbiturate is a rare example of a barbiturate designer drug, possibly the only such compound encountered in recent years.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.

Eterobarb (Antilon) is a barbiturate derivative. It has mainly anticonvulsant action with less sedative effects than the closely related compound phenobarbital. It saw reasonable success in clinical trials, but is not in widespread medical use.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

Diberal, also known as 5-(1,3-Dimethylbutyl)-5-ethylbarbituric acid or DMBB, is an atypical barbiturate. This compound can be either convulsant or anticonvulsant depending on which enantiomer is used.















