Related Research Articles

Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis. HCC is the third leading cause of cancer-related deaths worldwide.
Liver tumors are abnormal growth of liver cells on or in the liver. Several distinct types of tumors can develop in the liver because the liver is made up of various cell types. Liver tumors can be classified as benign (non-cancerous) or malignant (cancerous) growths. They may be discovered on medical imaging, and the diagnosis is often confirmed with liver biopsy. Signs and symptoms of liver masses vary from being asymptomatic to patients presenting with an abdominal mass, hepatomegaly, abdominal pain, jaundice, or some other liver dysfunction. Treatment varies and is highly specific to the type of liver tumor.

Everolimus, sold under the brand name Afinitor among others, is a medication used as an immunosuppressant to prevent rejection of organ transplants and as a targeted therapy in the treatment of renal cell cancer and other tumours.
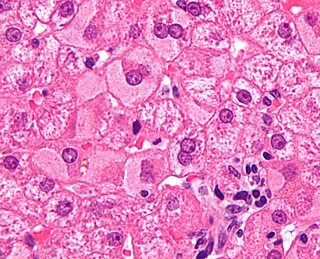
Viral hepatitis is liver inflammation due to a viral infection. It may present in acute form as a recent infection with relatively rapid onset, or in chronic form, typically progressing from a long-lasting asymptomatic condition up to a decompensated hepatic disease and hepatocellular carcinoma (HCC).
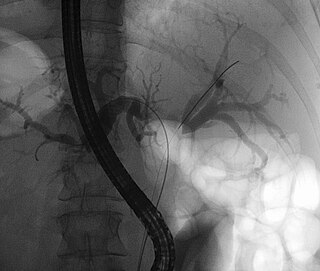
A Klatskin tumor is a cholangiocarcinoma occurring at the confluence of the right and left hepatic bile ducts. The disease was named after Gerald Klatskin, who in 1965 described 15 cases and found some characteristics for this type of cholangiocarcinoma
Hepatomegaly is enlargement of the liver. It is a non-specific medical sign, having many causes, which can broadly be broken down into infection, hepatic tumours, and metabolic disorder. Often, hepatomegaly presents as an abdominal mass. Depending on the cause, it may sometimes present along with jaundice.
Transcatheter arterial chemoembolization (TACE) is a minimally invasive procedure performed in interventional radiology to restrict a tumor's blood supply. Small embolic particles coated with chemotherapeutic drugs are injected selectively through a catheter into an artery directly supplying the tumor. These particles both block the blood supply and induce cytotoxicity, attacking the tumor in several ways.
In oncology, AFP-L3 is an isoform of alpha-fetoprotein (AFP), a substance typically used in the triple test during pregnancy and for screening chronic liver disease patients for hepatocellular carcinoma (HCC). AFP can be fractionated by affinity electrophoresis into three glycoforms: L1, L2, and L3 based on the reactivity with the lectin Lens culinaris agglutinin (LCA). AFP-L3 binds strongly to LCA via an additional α 1-6 fucose residue attached at the reducing terminus of N-acetylglucosamine; this is in contrast to the L1 isoform. It is the L1 isoform which is typically associated with non-HCC inflammation of liver disease condition. The L3 isoform is specific to malignant tumors and its detected presence can serve to identify patients whom need increased monitoring for the development of HCC in high risk populations. AFP-L3% is now being considered as a tumor marker for the North American demographic.

Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.
The Model for End-Stage Liver Disease, or MELD, is a scoring system for assessing the severity of chronic liver disease. It was initially developed to predict mortality within three months of surgery in patients who had undergone a transjugular intrahepatic portosystemic shunt (TIPS) procedure, and was subsequently found to be useful in determining prognosis and prioritizing for receipt of a liver transplant. This score is now used by the United Network for Organ Sharing (UNOS) and Eurotransplant for prioritizing allocation of liver transplants instead of the older Child-Pugh score.
Des-gamma carboxyprothrombin (DCP), also known as protein induced by vitamin K absence/antagonist-II (PIVKA-II), is an abnormal form of the coagulation protein, prothrombin. Normally, the prothrombin precursor undergoes post-translational carboxylation by gamma-glutamyl carboxylase in the liver prior to secretion into plasma. DCP/PIVKA-II may be detected in people with deficiency of vitamin K and in those taking warfarin or other medication that inhibits the action of vitamin K.

Liver cancer is cancer that starts in the liver. Liver cancer can be primary or secondary. Liver metastasis is more common than that which starts in the liver. Liver cancer is increasing globally.
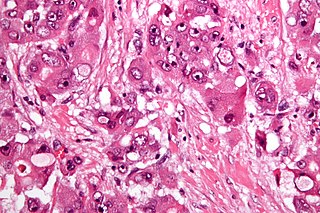
Fibrolamellar carcinoma (FLC) is a rare form of carcinoma that typically affects young adults and is characterized, under the microscope, by laminated fibrous layers interspersed between the tumor cells. It has been estimated that 200 new cases are diagnosed worldwide each year. However, in light of recent advances in our molecular understanding, this has recently been revised to suggest it may be at least ten times more common. FLC, also known as fibrolamellar hepatocellular carcinoma, is different from the more common hepatocellular carcinoma (HCC) in that it afflicts young people with normal liver function and no known risk factors.

Selective internal radiation therapy (SIRT), also known as transarterial radioembolization (TARE), radioembolization or intra-arterial microbrachytherapy is a form of radiation therapy used in interventional radiology to treat cancer. It is generally for selected patients with surgically unresectable cancers, especially hepatocellular carcinoma or metastasis to the liver. The treatment involves injecting tiny microspheres of radioactive material into the arteries that supply the tumor, where the spheres lodge in the small vessels of the tumor. Because this treatment combines radiotherapy with embolization, it is also called radioembolization. The chemotherapeutic analogue is called chemoembolization, of which transcatheter arterial chemoembolization (TACE) is the usual form.

Cirrhosis, also known as liver cirrhosis or hepatic cirrhosis, and end-stage liver disease, is the impaired liver function caused by the formation of scar tissue known as fibrosis due to damage caused by liver disease. Damage to the liver leads to repair of liver tissue and subsequent formation of scar tissue. Over time, scar tissue can replace normal functioning tissue, leading to the impaired liver function of cirrhosis. The disease typically develops slowly over months or years. Early symptoms may include tiredness, weakness, loss of appetite, unexplained weight loss, nausea and vomiting, and discomfort in the right upper quadrant of the abdomen. As the disease worsens, symptoms may include itchiness, swelling in the lower legs, fluid build-up in the abdomen, jaundice, bruising easily, and the development of spider-like blood vessels in the skin. The fluid build-up in the abdomen may develop into spontaneous infections. More serious complications include hepatic encephalopathy, bleeding from dilated veins in the esophagus, stomach, or intestines, and liver cancer.

Brivanib alaninate (INN/USAN) also known as BMS-582664 is an investigational, anti-tumorigenic drug for oral administration. The drug is being developed by Bristol-Myers Squibb for the treatment of hepatocellular carcinoma or HCC, the most common type of liver cancer. Brivanib is no longer in active development.

The Liver Imaging Reporting and Data System is a quality assurance tool created and trademarked by the American College of Radiology in 2011 to standardize the reporting and data collection of CT and MR imaging patients at risk for hepatocellular carcinoma (HCC), or primary cancer of the liver cells. It provides a standardized framework for classification of liver lesions by a radiologist, and only applies in patients with chronic liver disease, the main risk factor for liver cancer. The hierarchical classification, from LR1 to LR5, is based on specific imaging features of the lesion in question, and corresponds to the degree of suspicion for malignancy. For example, a lesion with features corresponding to the highest category, LR5, is "definitely" HCC. Importantly, the increasing acceptance of the LI-RADS system of reporting by referring clinicians has reduced the need for tissue biopsy confirmation of cancer in patients with chronic liver disease.
Ultrasonography of liver tumors involves two stages: detection and characterization.
Transarterial bland embolization is a catheter-based tumor treatment of the liver. In this procedure, a variety of embolizing agents can be delivered through the tumor’s feeding artery in order to completely occlude the tumor’s blood supply. The anti-tumor effects are solely based on tumor ischemia and infarction of tumor tissue, as no chemotherapeutic agents are administered. The rationale for the use of bland embolization for hepatocellular carcinoma(HCC) and/or other hyper-vascular tumors is based on the fact that normal liver receives a dual blood supply from the hepatic artery (25%) and the portal vein (75%). As the tumor grows, it becomes increasingly dependent on the hepatic artery for blood supply. Once a tumor nodule reaches a diameter of 2 cm or more, most of the blood supply is derived from the hepatic artery. Therefore, bland embolization and transarterial chemoembolization (TACE) consist of the selective angiographic occlusion of the tumor arterial blood supply with a variety of embolizing agents, with or without the precedence of local chemotherapy infusion. The occlusion by embolic particles results in tumor hypoxia and necrosis, without affecting the normal hepatic parenchyma.
Nancy L. Ascher is an American surgeon, and the first woman to perform a liver transplant. Ascher specializes in transplant surgery, focusing on end-stage kidney disease, kidney transplantation, non-alcoholic fatty liver disease and liver transplantation.
References
- ↑ Mazzaferro, Vincenzo; Regalia, Enrico; Doci, Roberto; Andreola, Salvatore; Pulvirenti, Andrea; Bozzetti, Federico; Montalto, Fabrizio; Ammatuna, Mario; Morabito, Alberto (1996-03-14). "Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis". New England Journal of Medicine. 334 (11): 693–700. doi: 10.1056/nejm199603143341104 . ISSN 0028-4793. PMID 8594428.
- ↑ Wald, Christoph; Russo, Mark W.; Heimbach, Julie K.; Hussain, Hero K.; Pomfret, Elizabeth A.; Bruix, Jordi (2013-02-01). "New OPTN/UNOS Policy for Liver Transplant Allocation: Standardization of Liver Imaging, Diagnosis, Classification, and Reporting of Hepatocellular Carcinoma". Radiology. 266 (2): 376–382. doi:10.1148/radiol.12121698. ISSN 0033-8419. PMID 23362092.
- ↑ Yao, F. Y. (October 2008). "Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria". American Journal of Transplantation. 8 (10): 1982–1989. doi: 10.1111/j.1600-6143.2008.02351.x . ISSN 1600-6143. PMID 18727702.
- ↑ Yao, Francis Y.; Ferrell, Linda; Bass, Nathan M.; Watson, Jessica J.; Bacchetti, Peter; Venook, Alan; Ascher, Nancy L.; Roberts, John P. (2001-06-01). "Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival". Hepatology. 33 (6): 1394–1403. doi: 10.1053/jhep.2001.24563 . ISSN 1527-3350. PMID 11391528.
- ↑ Duffy, John P.; Vardanian, Andrew; Benjamin, Elizabeth; Watson, Melissa; Farmer, Douglas G.; Ghobrial, Rafik M.; Lipshutz, Gerald; Yersiz, Hasan; Lu, David S. K. (2007). "Liver Transplantation Criteria For Hepatocellular Carcinoma Should Be Expanded". Annals of Surgery. 246 (3): 502–511. doi:10.1097/sla.0b013e318148c704. PMC 1959350 . PMID 17717454.
- ↑ Tang, An; Fowler, Kathryn J.; Chernyak, Victoria; Chapman, William C.; Sirlin, Claude B. (2017-06-13). "LI-RADS and transplantation for hepatocellular carcinoma". Abdominal Radiology. 43 (1): 193–202. doi:10.1007/s00261-017-1210-8. hdl: 1866/28271 . ISSN 2366-004X. PMID 28612162.