Related Research Articles
General anaesthetics are often defined as compounds that induce a loss of consciousness in humans or loss of righting reflex in animals. Clinical definitions are also extended to include an induced coma that causes lack of awareness to painful stimuli, sufficient to facilitate surgical applications in clinical and veterinary practice. General anaesthetics do not act as analgesics and should also not be confused with sedatives. General anaesthetics are a structurally diverse group of compounds whose mechanisms encompass multiple biological targets involved in the control of neuronal pathways. The precise workings are the subject of some debate and ongoing research.

Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.

Isoflurane, sold under the brand name Forane among others, is a general anesthetic. It can be used to start or maintain anesthesia; however, other medications are often used to start anesthesia, due to airway irritation with isoflurane. Isoflurane is given via inhalation.

Sevoflurane, sold under the brand name Sevorane, among others, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desflurane, it is the volatile anesthetic with the fastest onset. While its offset may be faster than agents other than desflurane in a few circumstances, its offset is more often similar to that of the much older agent isoflurane. While sevoflurane is only half as soluble as isoflurane in blood, the tissue blood partition coefficients of isoflurane and sevoflurane are quite similar. For example, in the muscle group: isoflurane 2.62 vs. sevoflurane 2.57. In the fat group: isoflurane 52 vs. sevoflurane 50. As a result, the longer the case, the more similar will be the emergence times for sevoflurane and isoflurane.

Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance.

General anaesthesia (UK) or general anesthesia (US) is a method of medically inducing loss of consciousness that renders a patient unarousable even with painful stimuli. This effect is achieved by administering either intravenous or inhalational general anaesthetic medications, which often act in combination with an analgesic and neuromuscular blocking agent. Spontaneous ventilation is often inadequate during the procedure and intervention is often necessary to protect the airway. General anaesthesia is generally performed in an operating theater to allow surgical procedures that would otherwise be intolerably painful for a patient, or in an intensive care unit or emergency department to facilitate endotracheal intubation and mechanical ventilation in critically ill patients.

Propofol is the active component of an intravenous anesthetic formulation used for induction and maintenance of general anesthesia. It is chemically termed 2,6-diisopropylphenol. The formulation was originally approved under the brand name Diprivan. Numerous generic offerings of this formulation now exist. Intravenous administration is used to induce unconsciousness after which anesthesia may be maintained using a combination of medications. It is manufactured as part of a sterile injectable emulsion formulation using soybean oil and lecithin, giving it a white milky coloration.

A general anaesthetic is a drug that brings about a reversible loss of consciousness. These drugs are generally administered by an anaesthetist/anesthesiologist to induce or maintain general anaesthesia to facilitate surgery.

An anesthetic or anaesthetic is a drug used to induce anesthesia — in other words, to result in a temporary loss of sensation or awareness. They may be divided into two broad classes: general anesthetics, which result in a reversible loss of consciousness, and local anesthetics, which cause a reversible loss of sensation for a limited region of the body without necessarily affecting consciousness.
Awareness under anesthesia, also referred to as intraoperative awareness or accidental awareness during general anesthesia (AAGA), is a rare complication of general anesthesia where patients regain varying levels of consciousness during their surgical procedures. While anesthesia awareness is possible without resulting in any long-term memory of the experience, it is also possible for victims to have awareness with explicit recall, where they can remember the events related to their surgery.
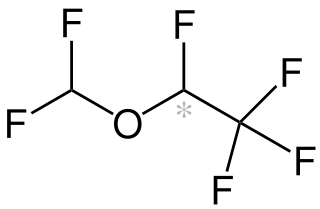
Desflurane (1,2,2,2-tetrafluoroethyl difluoromethyl ether) is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane, and isoflurane, it is a racemic mixture of (R) and (S) optical isomers (enantiomers). Together with sevoflurane, it is gradually replacing isoflurane for human use, except in economically undeveloped areas, where its high cost precludes its use. It has the most rapid onset and offset of the volatile anesthetic drugs used for general anesthesia due to its low solubility in blood.

Malignant hyperthermia (MH) is a type of severe reaction that occurs in response to particular medications used during general anesthesia, among those who are susceptible. Symptoms include muscle rigidity, fever, and a fast heart rate. Complications can include muscle breakdown and high blood potassium. Most people who are susceptible to MH are generally unaffected when not exposed to triggering agents.

Remifentanil, marketed under the brand name Ultiva is a potent, short-acting synthetic opioid analgesic drug. It is given to patients during surgery to relieve pain and as an adjunct to an anaesthetic. Remifentanil is used for sedation as well as combined with other medications for use in general anesthesia. The use of remifentanil has made possible the use of high-dose opioid and low-dose hypnotic anesthesia, due to synergism between remifentanil and various hypnotic drugs and volatile anesthetics.

An inhalational anesthetic is a chemical compound possessing general anesthetic properties that is delivered via inhalation. They are administered through a face mask, laryngeal mask airway or tracheal tube connected to an anesthetic vaporiser and an anesthetic delivery system. Agents of significant contemporary clinical interest include volatile anesthetic agents such as isoflurane, sevoflurane and desflurane, as well as certain anesthetic gases such as nitrous oxide and xenon.

Methoxyflurane, sold under the brand name Penthrox among others, is an inhaled medication primarily used to reduce pain following trauma. It may also be used for short episodes of pain as a result of medical procedures. Onset of pain relief is rapid and of a short duration. Use is only recommended with direct medical supervision.

Flurothyl (Indoklon) is a volatile liquid drug from the halogenated ether family, related to inhaled anaesthetic agents such as diethyl ether, but having the opposite effects, acting as a stimulant and convulsant. A clear and stable liquid, it has a mild ethereal odor whose vapors are non-flammable. It is excreted from the body by the lungs in an unchanged state.

An anesthetic vaporizer or anaesthetic vaporiser is a device generally attached to an anesthetic machine which delivers a given concentration of a volatile anesthetic agent. It works by controlling the vaporization of anesthetic agents from liquid, and then accurately controlling the concentration in which these are added to the fresh gas flow. The design of these devices takes account of varying: ambient temperature, fresh gas flow, and agent vapor pressure.
Emergence delirium is a condition in which emergence from general anesthesia is accompanied by psychomotor agitation. Some see a relation to pavor nocturnus while others see a relation to the excitement stage of anesthesia.
Blood–gas partition coefficient, also known as Ostwald coefficient for blood–gas, is a term used in pharmacology to describe the solubility of inhaled general anesthetics in blood. According to Henry's law, the ratio of the concentration in blood to the concentration in gas that is in contact with that blood, when the partial pressure in both compartments is equal, is nearly constant at sufficiently low concentrations. The partition coefficient is defined as this ratio and, therefore, has no units. The concentration of the anesthetic in blood includes the portion that is undissolved in plasma and the portion that is dissolved. The more soluble the inhaled anesthetic is in blood compared to in air, the more it binds to plasma proteins in the blood and the higher the blood–gas partition coefficient.
The effects of early-life exposures to anesthesia on the brain in humans are controversial. Evidence from nonhuman primate research suggests significant developmental neurotoxicity and long-term social impairment, with a dose–response relationship where repeated exposures cause a more severe impact than single ones. Research in humans has not found conclusive clinical evidence of cognitive impairment; however, systematic reviews imply the possibility of greater behavioural impairments in children exposed to anesthesia before the age of three than control subjects.
References
- ↑ "Policy: Ban on Use of Ether". Laboratory Animal Science Center. Archived from the original on 2008-06-09. Retrieved 2008-11-10.
- ↑ Eger EI, Saidman LJ, Brandstater B (1965). "Minimum alveolar anesthetic concentration: a standard of anesthetic potency". Anesthesiology. 26 (6): 756–63. doi: 10.1097/00000542-196511000-00010 . PMID 5844267.
- ↑ Merkel, Giles; Eger, Edmond I. (1963-05-01). "A Comparative Study of Halothane and Halopropane AnesthesiaIncluding Method for Determining Equipotency". The Journal of the American Society of Anesthesiologists. 24 (3): 346–357. doi: 10.1097/00000542-196305000-00016 . ISSN 0003-3022. PMID 13935000. S2CID 35750587.
- 1 2 Eger, Edmond I.; Saidman, Lawrence J.; Brandstater, Bernard (1965-11-01). "Minimum Alveolar Anesthetic ConcentrationA Standard of Anesthetic Potency". The Journal of the American Society of Anesthesiologists. 26 (6): 756–763. doi: 10.1097/00000542-196511000-00010 . ISSN 0003-3022. PMID 5844267.
- ↑ Miller ANESTHESIOLOGY
- ↑
- Daniel M, Weiskopf RB, Noorani M, Eger EI (January 1998). "Fentanyl augments the blockade of the sympathetic response to incision (MAC-BAR) produced by desflurane and isoflurane: desflurane and isoflurane MAC-BAR without and with fentanyl". Anesthesiology. 88 (1): 43–9. doi:10.1097/00000542-199801000-00009. PMID 9447854. S2CID 19866907.
- ↑ Katoh T, Kobayashi S, Suzuki A, Iwamoto T, Bito H, Ikeda K (February 1999). "The effect of fentanyl on sevoflurane requirements for somatic and sympathetic responses to surgical incision". Anesthesiology. 90 (2): 398–405. doi: 10.1097/00000542-199902000-00012 . PMID 9952144.
- 1 2 3 Lobo, S. A.; Ojeda, J.; Dua, A.; Singh, K.; Lopez, J. (2022). "Minimum Alveolar Concentration". StatPearls. PMID 30422569.
- ↑ Eger Ei, 2nd (2001). "Age, minimum alveolar anesthetic concentration, and minimum alveolar anesthetic concentration-awake". Anesthesia and Analgesia. 93 (4): 947–953. doi: 10.1097/00000539-200110000-00029 . PMID 11574362. S2CID 31218667.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - 1 2 3 4 5 6 7 8
- Nickalls, R. W. D., & Mapleson, W. W. (August 2003). "Age-related iso-MAC charts for isoflurane, sevoflurane, and desflurane in man". British Journal of Anaesthesia. 91 (2): 170–4. doi: 10.1093/bja/aeg132 . PMID 12878613.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- Nickalls, R. W. D., & Mapleson, W. W. (August 2003). "Age-related iso-MAC charts for isoflurane, sevoflurane, and desflurane in man". British Journal of Anaesthesia. 91 (2): 170–4. doi: 10.1093/bja/aeg132 . PMID 12878613.