
A transposable element is a nucleic acid sequence in DNA that can change its position within a genome, sometimes creating or reversing mutations and altering the cell's genetic identity and genome size. Transposition often results in duplication of the same genetic material. In the human genome, L1 and Alu elements are two examples. Barbara McClintock's discovery of them earned her a Nobel Prize in 1983. Its importance in personalized medicine is becoming increasingly relevant, as well as gaining more attention in data analytics given the difficulty of analysis in very high dimensional spaces.

A homeobox is a DNA sequence, around 180 base pairs long, that regulates large-scale anatomical features in the early stages of embryonic development. Mutations in a homeobox may change large-scale anatomical features of the full-grown organism.
Hox genes, a subset of homeobox genes, are a group of related genes that specify regions of the body plan of an embryo along the head-tail axis of animals. Hox proteins encode and specify the characteristics of 'position', ensuring that the correct structures form in the correct places of the body. For example, Hox genes in insects specify which appendages form on a segment, and Hox genes in vertebrates specify the types and shape of vertebrae that will form. In segmented animals, Hox proteins thus confer segmental or positional identity, but do not form the actual segments themselves.
Piwi-interacting RNA (piRNA) is the largest class of small non-coding RNA molecules expressed in animal cells. piRNAs form RNA-protein complexes through interactions with piwi-subfamily Argonaute proteins. These piRNA complexes are mostly involved in the epigenetic and post-transcriptional silencing of transposable elements and other spurious or repeat-derived transcripts, but can also be involved in the regulation of other genetic elements in germ line cells.

In molecular biology, mir-46 and mir-47 are microRNA expressed in C. elegans from related hairpin precursor sequences. The predicted hairpin precursor sequences for Drosophila mir-281 are also related and, hence, belong to this family. The hairpin precursors are predicted based on base pairing and cross-species conservation; their extents are not known. In this case, the mature sequences are expressed from the 3' arms of the hairpin precursors.

The miR-8 microRNA precursor, is a short non-coding RNA gene involved in gene regulation. miR-8 in Drosophila melanogaster is expressed from the 3' arm of related precursor hairpins, along with miR-200, miR-236, miR-429 and human and mouse homolog miR-141. Members of this precursor family have now been predicted or experimentally confirmed in a wide range of species. The bounds of the precursors are predicted based on conservation and base pairing and are not generally known.

The miR-9 microRNA, is a short non-coding RNA gene involved in gene regulation. The mature ~21nt miRNAs are processed from hairpin precursor sequences by the Dicer enzyme. The dominant mature miRNA sequence is processed from the 5' arm of the mir-9 precursor, and from the 3' arm of the mir-79 precursor. The mature products are thought to have regulatory roles through complementarity to mRNA. In vertebrates, miR-9 is highly expressed in the brain, and is suggested to regulate neuronal differentiation. A number of specific targets of miR-9 have been proposed, including the transcription factor REST and its partner CoREST.

The mir-10 microRNA precursor is a short non-coding RNA gene involved in gene regulation. It is part of an RNA gene family which contains mir-10, mir-51, mir-57, mir-99 and mir-100. mir-10, mir-99 and mir-100 have now been predicted or experimentally confirmed in a wide range of species. miR-51 and miR-57 have currently only been identified in the nematode Caenorhabditis elegans.

miR-196 is a non-coding RNA called a microRNA that has been shown to be expressed in humans and mice. miR-196 appears to be a vertebrate specific microRNA and has now been predicted or experimentally confirmed in a wide range of vertebrate species. In many species the miRNA appears to be expressed from intergenic regions in HOX gene clusters. The hairpin precursors are predicted based on base pairing and cross-species conservation—their extents are not known. In this case the mature sequence is excised from the 5' arm of the hairpin.

There are 89 known sequences today in the microRNA 19 (miR-19) family but it will change quickly. They are found in a large number of vertebrate species. The miR-19 microRNA precursor is a small non-coding RNA molecule that regulates gene expression. Within the human and mouse genome there are three copies of this microRNA that are processed from multiple predicted precursor hairpins:
The miR-34 microRNA precursor family are non-coding RNA molecules that, in mammals, give rise to three major mature miRNAs. The miR-34 family members were discovered computationally and later verified experimentally. The precursor miRNA stem-loop is processed in the cytoplasm of the cell, with the predominant miR-34 mature sequence excised from the 5' arm of the hairpin.

The mir-6 microRNA precursor is a precursor microRNA specific to Drosophila species. In Drosophila melanogaster there are three mir-6 paralogs called dme-mir-6-1, dme-mir-6-2, dme-mir-6-3, which are clustered together in the genome. The extents of these hairpin precursors are estimated based on hairpin prediction. Each precursor is generated following the cleavage of a longer primary transcript in the nucleus, and is exported in the cytoplasm. In the cytoplasm, precursors are further processed by the enzyme Dicer, generating ~22 nucleotide products from each arm of the hairpin. The products generated from the 3' arm of each mir-6 precursor have identical sequences. Both 5' and 3' mature products are experimentally validated. Experimental data suggests that the mature products of mir-6 hairpins are expressed in the early embryo of Drosophila and target apoptotic genes such as hid, grim and rpr.
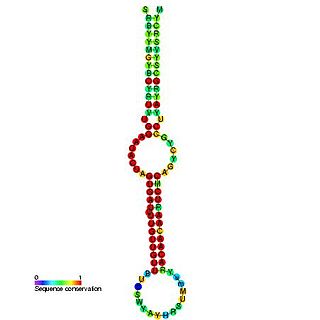
This family represents the microRNA (miRNA) precursor mir-7. This miRNA has been predicted or experimentally confirmed in a wide range of species. miRNAs are transcribed as ~70 nucleotide precursors and subsequently processed by the Dicer enzyme to give a ~22 nucleotide product. In this case the mature sequence comes from the 5' arm of the precursor. The extents of the hairpin precursors are not generally known and are estimated based on hairpin prediction. The involvement of Dicer in miRNA processing suggests a relationship with the phenomenon of RNA interference.

The miR-92 microRNAs are short single stranded non-protein coding RNA fragments initially discovered incorporated into an RNP complex with a proposed role of processing RNA molecules and further RNP assembly. Mir-92 has been mapped to the human genome as part of a larger cluster at chromosome 13q31.3, where it is 22 nucleotides in length but exists in the genome as part of a longer precursor sequence. There is an exact replica of the mir-92 precursor on the X chromosome. MicroRNAs are endogenous triggers of the RNAi pathway which involves several ribonucleic proteins (RNPs) dedicated to repressing mRNA molecules via translation inhibition and/or induction of mRNA cleavage. miRNAs are themselves matured from their long RNA precursors by ribonucleic proteins as part of a 2 step biogenesis mechanism involving RNA polymerase 2.

miR-96 microRNA precursor is a small non-coding RNA that regulates gene expression. microRNAs are transcribed as ~80 nucleotide precursors and subsequently processed by the Dicer enzyme to give a ~23 nucleotide products. In this case the mature sequence comes from the 5′ arm of the precursor. The mature products are thought to have regulatory roles through complementarity to mRNA.
Zerknüllt is a gene in the Antennapedia complex of Drosophila and other insects, where it operates very differently from the canonical Hox genes in the same gene cluster. Comparison of Hox genes between species showed that the Zerknüllt gene evolved from one of the standard Hox genes in insects through accumulating many amino acid changes, changing expression pattern, losing ancestral function and gaining a new function.
In molecular biology mir-14 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
In molecular biology mir-278 microRNA is a short RNA molecule belonging to a class of molecules referred to as microRNAs. These function to regulate the expression levels of other genes by several mechanisms, primarily binding to their target at its 3'UTR.
mir-279 is a short RNA molecule found in Drosophila melanogaster that belongs to a class of molecules known as microRNAs. microRNAs are ~22nt-long non-coding RNAs that post-transcriptionally regulate the expression of genes, often by binding to the 3' untranslated region of mRNA, targeting the transcript for degradation. miR-279 has diverse tissue-specific functions in the fly, influencing developmental processes related to neurogenesis and oogenesis, as well as behavioral processes related to circadian rhythms. The varied roles of mir-279, both in the developing and adult fly, highlight the utility of microRNAs in regulating unique biological processes.
In molecular biology mir-iab-4 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.














