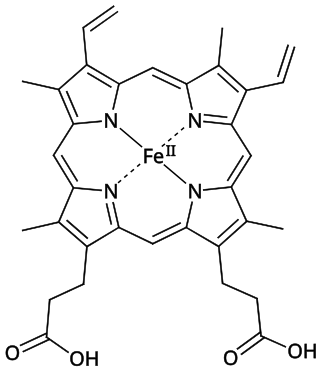
Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key component of the hemoglobin protein, acting as a transport medium for electrons within the cells in the form of cytochromes, and facilitating oxygen enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and death.

Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.
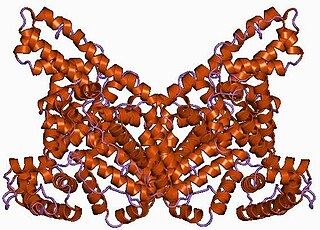
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule in order to form a glycoconjugate. In biology, glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation may refer to a non-enzymatic reaction.
An oligosaccharide is a saccharide polymer containing a small number of monosaccharides. Oligosaccharides can have many functions including cell recognition and cell adhesion.

Acute-phase proteins (APPs) are a class of proteins whose concentrations in blood plasma either increase or decrease in response to inflammation. This response is called the acute-phase reaction. The acute-phase reaction characteristically involves fever, acceleration of peripheral leukocytes, circulating neutrophils and their precursors. The terms acute-phase protein and acute-phase reactant (APR) are often used synonymously, although some APRs are polypeptides rather than proteins.

Lactoferrin (LF), also known as lactotransferrin (LTF), is a multifunctional protein of the transferrin family. Lactoferrin is a globular glycoprotein with a molecular mass of about 80 kDa that is widely represented in various secretory fluids, such as milk, saliva, tears, and nasal secretions. Lactoferrin is also present in secondary granules of PMNs and is secreted by some acinar cells. Lactoferrin can be purified from milk or produced recombinantly. Human colostrum has the highest concentration, followed by human milk, then cow milk (150 mg/L).
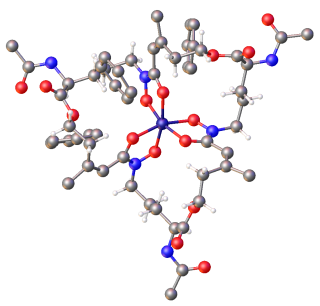
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated, siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.

Chromium(III) picolinate is a chemical compound with the formula Cr(C5H4N )3, commonly abbreviated as CrPic3. It is a bright-red coordination compound derived from chromium(III) and picolinic acid.

Human serum albumin is the serum albumin found in human blood. It is the most abundant protein in human blood plasma; it constitutes about half of serum protein. It is produced in the liver. It is soluble in water, and it is monomeric.
Albumin is a family of globular proteins, the most common of which are the serum albumins. All of the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins are called albuminoids.
Iron-binding proteins are carrier proteins and metalloproteins that are important in iron metabolism and the immune response. Iron is required for life.
Ovomucin is a glycoprotein found mainly in egg whites, as well as in the chalaza and vitelline membrane. The protein makes up around 2-4% of the protein content of egg whites; like other members of the mucin protein family, ovomucin confers gel-like properties. It is composed of two subunits, alpha-ovomucin (MUC5B) and beta-ovomucin (MUC6), of which the beta subunit is much more heavily glycosylated. The alpha subunit has a high number of acidic amino acids, while the beta subunit has more hydroxyl amino acids. The protein has a carbohydrate content of around 33%, featuring at least three unique types of carbohydrate side chains. It is known to possess a wide range of biological activities, including regulating cell functions and promoting the production of macrophages, lymphocytes, and cytokines, suggesting that it plays a role in the immune system.

Tej Pal Singh is an Indian biophysicist known for his work in the fields of rational structure-based drug design, structural biology of proteins and X-ray crystallography. He has played an active role in the development of drug design in the fields of antibacterial therapeutics, tuberculosis, inflammation, cancer and gastropathy.
Polysialic acid is an unusual posttranslational modification that occurs on neural cell adhesion molecules (NCAM). Polysialic acid is considerably anionic. This strong negative charge gives this modification the ability to change the protein's surface charge and binding ability. In the synapse, polysialation of NCAM prevents its ability to bind to NCAMs on the adjacent membrane.
Feline coronavirus (FCoV) is a positive-stranded RNA virus that infects cats worldwide. It is a coronavirus of the species Alphacoronavirus 1, which includes canine coronavirus (CCoV) and porcine transmissible gastroenteritis coronavirus (TGEV). FCoV has two different forms: feline enteric coronavirus (FECV), which infects the intestines, and feline infectious peritonitis virus (FIPV), which causes the disease feline infectious peritonitis (FIP).

Intelectins are lectins expressed in humans and other chordates. Humans express two types of intelectins encoded by ITLN1 and ITLN2 genes respectively. Several intelectins bind microbe-specific carbohydrate residues. Therefore, intelectins have been proposed to function as immune lectins. Even though intelectins contain fibrinogen-like domain found in the ficolins family of immune lectins, there is significant structural divergence. Thus, intelectins may not function through the same lectin-complement pathway. Most intelectins are still poorly characterized and they may have diverse biological roles. Human intelectin-1 (hIntL-1) has also been shown to bind lactoferrin, but the functional consequence has yet to be elucidated. Additionally, hIntL-1 is a major component of asthmatic mucus and may be involved in insulin physiology as well.

Glycan-Protein interactions represent a class of biomolecular interactions that occur between free or protein-bound glycans and their cognate binding partners. Intramolecular glycan-protein (protein-glycan) interactions occur between glycans and proteins that they are covalently attached to. Together with protein-protein interactions, they form a mechanistic basis for many essential cell processes, especially for cell-cell interactions and host-cell interactions. For instance, SARS-CoV-2, the causative agent of COVID-19, employs its extensively glycosylated spike (S) protein to bind to the ACE2 receptor, allowing it to enter host cells. The spike protein is a trimeric structure, with each subunit containing 22 N-glycosylation sites, making it an attractive target for vaccine search.
GlycoRNAs are small non-coding RNAs with sialylated glycans.















