
Neomycin is an aminoglycoside antibiotic that displays bactericidal activity against gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against gram-positive bacilli and anaerobic gram-negative bacilli. Neomycin comes in oral and topical formulations, including creams, ointments, and eyedrops. Neomycin belongs to the aminoglycoside class of antibiotics that contain two or more amino sugars connected by glycosidic bonds.

Kanamycin A, often referred to simply as kanamycin, is an antibiotic used to treat severe bacterial infections and tuberculosis. It is not a first line treatment. It is used by mouth, injection into a vein, or injection into a muscle. Kanamycin is recommended for short-term use only, usually from 7 to 10 days. As with most antibiotics, it is ineffective in viral infections.
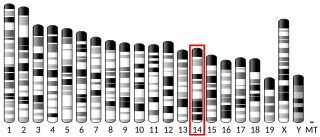
L-Gulonolactone oxidase is an enzyme that produces vitamin C, but is non-functional in Haplorrhini, in some bats, and in guinea pigs. It catalyzes the reaction of L-gulono-1,4-lactone with oxygen to form L-xylo-hex-3-gulonolactone (2-keto-gulono-γ-lactone) and hydrogen peroxide. It uses FAD as a cofactor. The L-xylo-hex-3-gulonolactone then converts to ascorbic acid spontaneously, without enzymatic action.

Isopenicillin N synthase (IPNS) is a non-heme iron protein belonging to the 2-oxoglutarate (2OG)-dependent dioxygenases oxidoreductase family. This enzyme catalyzes the formation of isopenicillin N from δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine (LLD-ACV).
In enzymology, a reticuline oxidase (EC 1.21.3.3) is an enzyme that catalyzes the chemical reaction
Long-chain alcohol oxidase is one of two enzyme classes that oxidize long-chain or fatty alcohols to aldehydes. It has been found in certain Candida yeast, where it participates in omega oxidation of fatty acids to produce acyl-CoA for energy or industrial use, as well as in other fungi, plants, and bacteria.

Ribostamycin is an aminoglycoside-aminocyclitol antibiotic isolated from a streptomycete, Streptomyces ribosidificus, originally identified in a soil sample from Tsu City of Mie Prefecture in Japan. It is made up of 3 ring subunits: 2-deoxystreptamine (DOS), neosamine C, and ribose. Ribostamycin, along with other aminoglycosides with the DOS subunit, is an important broad-spectrum antibiotic with important use against human immunodeficiency virus and is considered a critically important antimicrobial by the World Health Organization., Resistance against aminoglycoside antibiotics, such as ribostamycin, is a growing concern. The resistant bacteria contain enzymes that modify the structure through phosphorylation, adenylation, and acetylation and prevent the antibiotic from being able to interact with the bacterial ribosomal RNAs.

Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as Streptomyces azureus and Streptomyces laurentii. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class.
Radical SAM is a designation for a superfamily of enzymes that use a [4Fe-4S]+ cluster to reductively cleave S-adenosyl-L-methionine (SAM) to generate a radical, usually a 5′-deoxyadenosyl radical (5'-dAdo), as a critical intermediate. These enzymes utilize this radical intermediate to perform diverse transformations, often to functionalize unactivated C-H bonds. Radical SAM enzymes are involved in cofactor biosynthesis, enzyme activation, peptide modification, post-transcriptional and post-translational modifications, metalloprotein cluster formation, tRNA modification, lipid metabolism, biosynthesis of antibiotics and natural products etc. The vast majority of known radical SAM enzymes belong to the radical SAM superfamily, and have a cysteine-rich motif that matches or resembles CxxxCxxC. rSAMs comprise the largest superfamily of metal-containing enzymes.
2-deoxy-scyllo-inosamine dehydrogenase (EC 1.1.1.329, neoA (gene name), kanK (gene name)) is an enzyme with systematic name 2-deoxy-scyllo-inosamine:NAD(P)+ 1-oxidoreductase. This enzyme catalyses the following chemical reaction
6'''-hydroxyneomycin C oxidase (EC 1.1.3.44, neoG (gene)) is an enzyme with systematic name 6'''-deamino-6'''-hydroxyneomycin C:oxygen 6'''-oxidoreductase. This enzyme catalyses the following chemical reaction
2-deoxy-scyllo-inosamine dehydrogenase (SAM-dependent) is an enzyme with systematic name 2-deoxy-scyllo-inosamine:S-adenosyl-L-methionine 1-oxidoreductase. This enzyme catalyses the following chemical reaction
2-deoxystreptamine N-acetyl-D-glucosaminyltransferase is an enzyme with systematic name UDP-N-acetyl-alpha-D-glucosamine:2-deoxystreptamine N-acetyl-D-glucosaminyltransferase. This enzyme catalyses the following chemical reaction
UDP-GlcNAc:ribostamycin N-acetylglucosaminyltransferase is an enzyme with systematic name UDP-N-acetyl-alpha-D-glucosamine:ribostamycin N-acetylglucosaminyltransferase. This enzyme catalyses the following chemical reaction
Neamine transaminase is an enzyme with systematic name neamine:2-oxoglutarate aminotransferase. This enzyme catalyses the following chemical reaction
Neomycin C transaminase is an enzyme with systematic name 2-oxoglutarate:neomycin C aminotransferase. This enzyme catalyses the following chemical reaction
2'-N-acetylparomamine deacetylase (EC 3.5.1.112, btrD (gene), neoL (gene), kanN (gene)) is an enzyme with systematic name 2'-N-acetylparomamine hydrolase (acetate-forming). This enzyme catalyses the following chemical reaction
2'''-acetyl-6'''-hydroxyneomycin C deacetylase (EC 3.5.1.113, neoL (gene)) is an enzyme with systematic name 2'''-acetyl-6'''-hydroxyneomycin C hydrolase (acetate-forming). This enzyme catalyses the following chemical reaction
2-deoxy-scyllo-Inosose synthase is an enzyme with systematic name D-glucose-6-phosphate phosphate-lyase (2-deoxy-scyllo-inosose-forming). This enzyme catalyses the following chemical reaction
γ-L-Glutamyl-butirosin B γ-glutamyl cyclotransferase is an enzyme with systematic name γ-L-glutamyl-butirosin B γ-glutamyl cyclotransferase . This enzyme catalyses the following chemical reaction






