Aciclovir, also known as acyclovir, is an antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein–Barr virus infection. It can be taken by mouth, applied as a cream, or injected.

Valaciclovir, also spelled valacyclovir, is an antiviral medication used to treat outbreaks of herpes simplex or herpes zoster (shingles). It is also used to prevent cytomegalovirus following a kidney transplant in high risk cases. It is taken by mouth.

Ganciclovir, sold under the brand name Cytovene among others, is an antiviral medication used to treat cytomegalovirus (CMV) infections.

Viral encephalitis is inflammation of the brain parenchyma, called encephalitis, by a virus. The different forms of viral encephalitis are called viral encephalitides. It is the most common type of encephalitis and often occurs with viral meningitis. Encephalitic viruses first cause infection and replicate outside of the central nervous system (CNS), most reaching the CNS through the circulatory system and a minority from nerve endings toward the CNS. Once in the brain, the virus and the host's inflammatory response disrupt neural function, leading to illness and complications, many of which frequently are neurological in nature, such as impaired motor skills and altered behavior.

Brivudine is an antiviral drug used in the treatment of herpes zoster ("shingles"). Like other antivirals, it acts by inhibiting replication of the target virus.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

Genital herpes is a herpes infection of the genitals caused by the herpes simplex virus (HSV). Most people either have no or mild symptoms and thus do not know they are infected. When symptoms do occur, they typically include small blisters that break open to form painful ulcers. Flu-like symptoms, such as fever, aching, or swollen lymph nodes, may also occur. Onset is typically around 4 days after exposure with symptoms lasting up to 4 weeks. Once infected further outbreaks may occur but are generally milder.
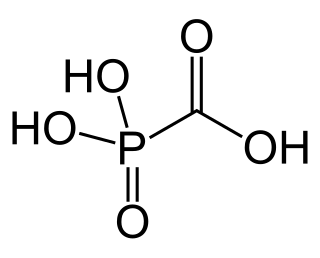
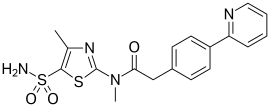
Foscarnet (phosphonomethanoic acid), known by its brand name Foscavir, is an antiviral medication which is primarily used to treat viral infections involving the Herpesviridae family. It is classified as a pyrophosphate analog DNA polymerase inhibitor. Foscarnet is the conjugate base of a chemical compound with the formula HO2CPO3H2 (Trisodium phosphonoformate).
Herpes gladiatorum is one of the most infectious of herpes-caused diseases, and is transmissible by skin-to-skin contact. The disease was first described in the 1960s in the New England Journal of Medicine. It is caused by contagious infection with human herpes simplex virus type 1 (HSV-1), which more commonly causes oral herpes. Another strain, HSV-2 usually causes genital herpes, although the strains are very similar and either can cause herpes in any location.
Lawrence Corey is an American immunologist and virologist known for his work in the development of antiviral therapies and vaccines, particularly for herpes simplex virus (HSV) and HIV/AIDS.

Mollaret's meningitis is a recurrent or chronic inflammation of the protective membranes covering the brain and spinal cord, known collectively as the meninges. Since Mollaret's meningitis is a recurrent, benign (non-cancerous), aseptic meningitis, it is also referred to as benign recurrent lymphocytic meningitis. It was named for Pierre Mollaret, the French neurologist who first described it in 1944.

Herpes simplex, often known simply as herpes, is a viral infection caused by the herpes simplex virus. Herpes infections are categorized by the area of the body that is infected. The two major types of herpes are oral herpes and genital herpes, though other forms also exist.
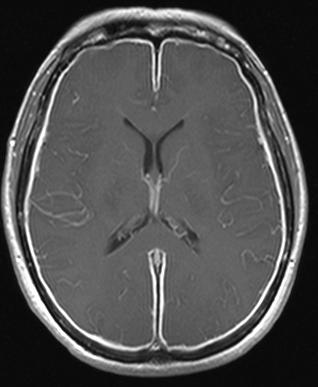
Herpes meningitis is inflammation of the meninges, the protective tissues surrounding the spinal cord and brain, due to infection from viruses of the Herpesviridae family - the most common amongst adults is HSV-2. Symptoms are self-limiting over 2 weeks with severe headache, nausea, vomiting, neck-stiffness, and photophobia. Herpes meningitis can cause Mollaret's meningitis, a form of recurrent meningitis. Lumbar puncture with cerebrospinal fluid results demonstrating aseptic meningitis pattern is necessary for diagnosis and polymerase chain reaction is used to detect viral presence. Although symptoms are self-limiting, treatment with antiviral medication may be recommended to prevent progression to Herpes Meningoencephalitis.

Herpes simplex encephalitis (HSE), or simply herpes encephalitis, is encephalitis due to herpes simplex virus. It is estimated to affect at least 1 in 500,000 individuals per year, and some studies suggest an incidence rate of 5.9 cases per 100,000 live births.

A cold sore is a type of herpes infection caused by the herpes simplex virus that affects primarily the lip. Symptoms typically include a burning pain followed by small blisters or sores. The first attack may also be accompanied by fever, sore throat, and enlarged lymph nodes. The rash usually heals within ten days, but the virus remains dormant in the trigeminal ganglion. The virus may periodically reactivate to create another outbreak of sores in the mouth or lip.

Herpes esophagitis is a viral infection of the esophagus caused by Herpes simplex virus (HSV).
Herpes simplex research includes all medical research that attempts to prevent, treat, or cure herpes, as well as fundamental research about the nature of herpes. Examples of particular herpes research include drug development, vaccines and genome editing. HSV-1 and HSV-2 are commonly thought of as oral and genital herpes respectively, but other members in the herpes family include chickenpox (varicella/zoster), cytomegalovirus, and Epstein-Barr virus. There are many more virus members that infect animals other than humans, some of which cause disease in companion animals or have economic impacts in the agriculture industry.

Herpetic simplex keratitis is a form of keratitis caused by recurrent herpes simplex virus (HSV) infection in the cornea.
A helicase–primase complex is a complex of enzymes including DNA helicase and DNA primase. A helicase-primase associated factor protein may also be present.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.