 | |
| Clinical data | |
|---|---|
| Trade names | Tpoxx |
| Other names | ST-246 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
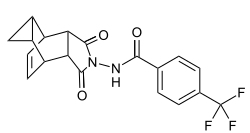
| Formula | C19H15F3N2O3 |
| Molar mass | 376.335 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tecovirimat, sold under the brand name Tpoxx among others, [6] is an antiviral medication with activity against orthopoxviruses such as smallpox and mpox. [4] [7] [8] In 2018 it was the first antipoxviral drug approved in the United States.
Contents
- Medical uses
- Mechanism of action
- Chemistry
- History
- Clinical trials
- Society and culture
- Legal status
- References
- External links
The drug works by blocking cellular transmission of orthopoxviruses, thus preventing disease. [9]
Tecovirimat has been effective in laboratory testing; it has been shown to protect animals from mpox and rabbitpox and causes no serious side effects in humans. [6] Tecovirimat was first used for treatment in December 2018, after a laboratory-acquired vaccinia virus infection. [10]
As of 2014 two million doses of tecovirimat were stockpiled in the US Strategic National Stockpile should an orthopoxvirus-based bioterror attack occur. [11] [12] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication. [13]
