
Fusarium ear blight (FEB), is a fungal disease of cereals, including wheat, barley, oats, rye and triticale. FEB is caused by a range of Fusarium fungi, which infects the heads of the crop, reducing grain yield. The disease is often associated with contamination by mycotoxins produced by the fungi already when the crop is growing in the field. The disease can cause severe economic losses as mycotoxin-contaminated grain cannot be sold for food or feed.

Corn smut is a plant disease caused by the pathogenic fungus Ustilago maydis. One of several cereal crop pathogens called smut, the fungus forms galls on all above-ground parts of corn species such as maize and teosinte. The infected corn is edible; in Mexico, it is considered a delicacy, called huitlacoche, often eaten as a filling in quesadillas and other tortilla-based dishes, as well as in soups.

The smuts are multicellular fungi characterized by their large numbers of teliospores. The smuts get their name from a Germanic word for 'dirt' because of their dark, thick-walled, and dust-like teliospores. They are mostly Ustilaginomycetes and comprise seven of the 15 orders of the subphylum. Most described smuts belong to two orders, Ustilaginales and Tilletiales. The smuts are normally grouped with the other basidiomycetes because of their commonalities concerning sexual reproduction.

Fusarium culmorum is a fungal plant pathogen and the causal agent of seedling blight, foot rot, ear blight, stalk rot, common root rot and other diseases of cereals, grasses, and a wide variety of monocots and dicots. In coastal dunegrass, F. culmorum is a nonpathogenic symbiont conferring both salt and drought tolerance to the plant.
Glomerella graminicola is an economically important crop parasite affecting both wheat and maize where it causes the plant disease Anthracnose Leaf Blight.

Gibberella zeae, also known by the name of its anamorph Fusarium graminearum, is a fungal plant pathogen which causes fusarium head blight (FHB), a devastating disease on wheat and barley. The pathogen is responsible for billions of dollars in economic losses worldwide each year. Infection causes shifts in the amino acid composition of wheat, resulting in shriveled kernels and contaminating the remaining grain with mycotoxins, mainly deoxynivalenol (DON), which inhibits protein biosynthesis; and zearalenone, an estrogenic mycotoxin. These toxins cause vomiting, liver damage, and reproductive defects in livestock, and are harmful to humans through contaminated food. Despite great efforts to find resistance genes against F. graminearum, no completely resistant variety is currently available. Research on the biology of F. graminearum is directed towards gaining insight into more details about the infection process and reveal weak spots in the life cycle of this pathogen to develop fungicides that can protect wheat from scab infection.

Sclerotinia sclerotiorum is a plant pathogenic fungus and can cause a disease called white mold if conditions are conducive. S. sclerotiorum can also be known as cottony rot, watery soft rot, stem rot, drop, crown rot and blossom blight. A key characteristic of this pathogen is its ability to produce black resting structures known as sclerotia and white fuzzy growths of mycelium on the plant it infects. These sclerotia give rise to a fruiting body in the spring that produces spores in a sac which is why fungi in this class are called sac fungi (Ascomycota). This pathogen can occur on many continents and has a wide host range of plants. When S. sclerotiorum is onset in the field by favorable environmental conditions, losses can be great and control measures should be considered.
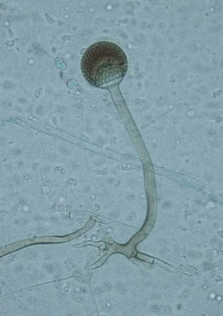
Rhizopus microsporus is a fungal plant pathogen infecting maize, sunflower, and rice.
Sporisorium reilianum Langdon & Full., (1978), previously known as Sphacelotheca reiliana, and Sporisorium reilianum, is a species of biotrophic fungus in the family Ustilaginaceae. It is a plant pathogen that infects maize and sorghum.

Cercospora sojina is a fungal plant pathogen which causes frogeye leaf spot of soybeans. Frog eye leaf spot is a major disease on soybeans in the southern U.S. and has recently started to expand into the northern U.S. where soybeans are grown. The disease is also found in other soybean production areas of the world.
Diaporthe phaseolorum var. caulivora is a fungal plant pathogen which infects soybean, causing soybean stem canker.

Maize, also known as corn in North American and Australian English, is a tall stout grass that produces cereal grain. It was domesticated by indigenous peoples in southern Mexico about 9,000 years ago from wild teosinte. Native Americans planted it alongside beans and squashes in the Three Sisters polyculture. The leafy stalk of the plant gives rise to male inflorescences or tassels which produce pollen, and female inflorescences called ears which yield grain, known as kernels or seeds. In modern commercial varieties, these are usually yellow or white; other varieties can be of many colors.
This article summarizes different crops, what common fungal problems they have, and how fungicide should be used in order to mitigate damage and crop loss. This page also covers how specific fungal infections affect crops present in the United States.

Grey leaf spot (GLS) is a foliar fungal disease that affects maize, also known as corn. GLS is considered one of the most significant yield-limiting diseases of corn worldwide. There are two fungal pathogens that cause GLS: Cercospora zeae-maydis and Cercospora zeina. Symptoms seen on corn include leaf lesions, discoloration (chlorosis), and foliar blight. Distinct symptoms of GLS are rectangular, brown to gray necrotic lesions that run parallel to the leaf, spanning the spaces between the secondary leaf veins. The fungus survives in the debris of topsoil and infects healthy crops via asexual spores called conidia. Environmental conditions that best suit infection and growth include moist, humid, and warm climates. Poor airflow, low sunlight, overcrowding, improper soil nutrient and irrigation management, and poor soil drainage can all contribute to the propagation of the disease. Management techniques include crop resistance, crop rotation, residue management, use of fungicides, and weed control. The purpose of disease management is to prevent the amount of secondary disease cycles as well as to protect leaf area from damage prior to grain formation. Corn grey leaf spot is an important disease of corn production in the United States, economically significant throughout the Midwest and Mid-Atlantic regions. However, it is also prevalent in Africa, Central America, China, Europe, India, Mexico, the Philippines, northern South America, and Southeast Asia. The teleomorph of Cercospora zeae-maydis is assumed to be Mycosphaerella sp.

Southern corn leaf blight (SCLB) is a fungal disease of maize caused by the plant pathogen Bipolaris maydis.
Stem rot is a disease caused by a fungus infection in the stem of crop plants. Fungus that causes stem rot are in the Rhizoctonia, Fusarium or Pythium genera. Stem rot can readily infect crops that are in their vegetative or flowering stages. The disease can survive up to five years in the soil. Symptoms of stem rot includes staining of infected area, reduced crop yield and crop failure. The disease can be spread through the use of unfiltered water as well as unsterilized tools. Also leaving previous dead roots in soil can increase the risk of stem rot. Spores can also enter the plant through injured stem tissue on the plant including from insect attacks. The fungus impedes stem functions like transporting nutrients. It can cause water to leak through the lesions of stem tissue. Common infected crop plants are soybeans and potatoes. An issue with maintaining this disease is the lack of management by crop producers. Producers of soybeans tend to not manage for the disease because it is not normally yield limiting in a large area. Fungicides can be used to manage the disease as well as burning the crop after harvest or letting it decompose.

Northern corn leaf blight (NCLB) or Turcicum leaf blight (TLB) is a foliar disease of corn (maize) caused by Exserohilum turcicum, the anamorph of the ascomycete Setosphaeria turcica. With its characteristic cigar-shaped lesions, this disease can cause significant yield loss in susceptible corn hybrids.
Cladosporium ear rot is a disease that affects maize. The disease is caused by the saprophytic fungus Cladosporium herbarum and is characterized by black or dark green fungal growths that cause black streaks on kernels.

Puccinia sorghi, or common rust of maize, is a species of rust fungus that infects corn and species from the plant genus Oxalis.
Phyllachora maydis is a plant pathogen causing ascomycete diseases in maize/corn, and is more commonly referred to as tar spot. Identified by the distinctive development of stroma, this pathogen in itself is of little economic importance in the production of corn. However, the accompanying fungal infection of Monographella maydis, identified by “fish-eye” lesions, was claimed to cause significant foliar damage and subsequently yield reduction. As of 2021 there is insufficient information about this pathogen and its management.















