| Thebaine 6-O-demethylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.14.11.31 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
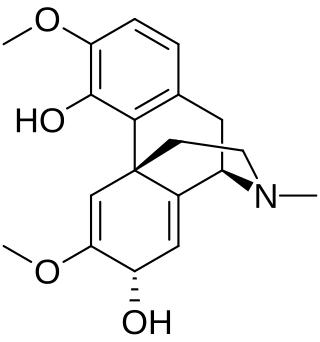
Thebaine 6-O-demethylase (EC 1.14.11.31, T6ODM) is an enzyme with systematic name thebaine,2-oxoglutarate:oxygen oxidoreductase (6-O-demethylating). [1] This enzyme catalyses the following chemical reaction
- thebaine + 2-oxoglutarate + O2 neopinone + formaldehyde + succinate + CO2
Thebaine 6-O-demethylase contains Fe2+.