Neutropenia is an abnormally low concentration of neutrophils in the blood. Neutrophils make up the majority of circulating white blood cells and serve as the primary defense against infections by destroying bacteria, bacterial fragments and immunoglobulin-bound viruses in the blood. People with neutropenia are more susceptible to bacterial infections and, without prompt medical attention, the condition may become life-threatening.
Bone marrow suppression also known as myelotoxicity or myelosuppression, is the decrease in production of cells responsible for providing immunity (leukocytes), carrying oxygen (erythrocytes), and/or those responsible for normal blood clotting (thrombocytes). Bone marrow suppression is a serious side effect of chemotherapy and certain drugs affecting the immune system such as azathioprine. The risk is especially high in cytotoxic chemotherapy for leukemia. In the case of non-small-cell lung cancer, myelosuppression predisposition was shown to be modulated by enhancer mutations.

The stromal cell-derived factor 1 (SDF-1), also known as C-X-C motif chemokine 12 (CXCL12), is a chemokine protein that in humans is encoded by the CXCL12 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types. Stromal cell-derived factors 1-alpha and 1-beta are small cytokines that belong to the chemokine family, members of which activate leukocytes and are often induced by proinflammatory stimuli such as lipopolysaccharide, TNF, or IL1. The chemokines are characterized by the presence of 4 conserved cysteines that form 2 disulfide bonds. They can be classified into 2 subfamilies. In the CC subfamily, the cysteine residues are adjacent to each other. In the CXC subfamily, they are separated by an intervening amino acid. The SDF1 proteins belong to the latter group. CXCL12 signaling has been observed in several cancers. The CXCL12 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.
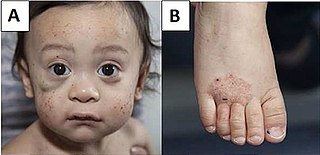
Wiskott–Aldrich syndrome (WAS) is a rare X-linked recessive disease characterized by eczema, thrombocytopenia, immune deficiency, and bloody diarrhea. It is also sometimes called the eczema-thrombocytopenia-immunodeficiency syndrome in keeping with Aldrich's original description in 1954. The WAS-related disorders of X-linked thrombocytopenia (XLT) and X-linked congenital neutropenia (XLN) may present with similar but less severe symptoms and are caused by mutations of the same gene.

C-C chemokine receptor type 5, also known as CCR5 or CD195, is a protein on the surface of white blood cells that is involved in the immune system as it acts as a receptor for chemokines.
Myelokathexis is a congenital disorder of the white blood cells that causes severe, chronic leukopenia and neutropenia. The disorder is believed to be inherited in an autosomal dominant manner. Myelokathexis refers to retention (kathexis) of neutrophils in the bone marrow (myelo). The disorder shows prominent neutrophil morphologic abnormalities.

Cyclic neutropenia (CyN) is a rare hematologic disorder and form of congenital neutropenia that tends to occur approximately every three weeks and lasting for few days at a time due to changing rates of neutrophil production by the bone marrow. It causes a temporary condition with a low absolute neutrophil count and because the neutrophils make up the majority of circulating white blood cells it places the body at severe risk of inflammation and infection. In comparison to severe congenital neutropenia, it responds well to treatment with granulocyte colony-stimulating factor (filgrastim), which increases the neutrophil count, shortens the cycle length, as well decreases the severity and frequency of infections.

C-X-C chemokine receptor type 4 (CXCR-4) also known as fusin or CD184 is a protein that in humans is encoded by the CXCR4 gene. The protein is a CXC chemokine receptor.
Severe congenital neutropenia (SCN), also often known as Kostmann syndrome or disease, is a group of rare disorders that affect myelopoiesis, causing a congenital form of neutropenia, usually without other physical malformations. SCN manifests in infancy with life-threatening bacterial infections. It causes severe pyogenic infections. It can be caused by autosomal dominant inheritance of the ELANE gene, autosomal recessive inheritance of the HAX1 gene. There is an increased risk of leukemia and myelodysplastic cancers.
Hypogammaglobulinemia is an immune system disorder in which not enough gamma globulins are produced in the blood. This results in a lower antibody count, which impairs the immune system, increasing risk of infection. Hypogammaglobulinemia may result from a variety of primary genetic immune system defects, such as common variable immunodeficiency, or it may be caused by secondary effects such as medication, blood cancer, or poor nutrition, or loss of gamma globulins in urine, as in nonselective glomerular proteinuria. Patients with hypogammaglobulinemia have reduced immune function; important considerations include avoiding use of live vaccines, and take precautionary measures when traveling to regions with endemic disease or poor sanitation such as receiving immunizations, taking antibiotics abroad, drinking only safe or boiled water, arranging appropriate medical cover in advance of travel, and ensuring continuation of any immunoglobulin infusions needed.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.

Chemokine ligand 2 (CXCL2) is a small cytokine belonging to the CXC chemokine family that is also called macrophage inflammatory protein 2-alpha (MIP2-alpha), Growth-regulated protein beta (Gro-beta) and Gro oncogene-2 (Gro-2). CXCL2 is 90% identical in amino acid sequence as a related chemokine, CXCL1. This chemokine is secreted by monocytes and macrophages and is chemotactic for polymorphonuclear leukocytes and hematopoietic stem cells. The gene for CXCL2 is located on human chromosome 4 in a cluster of other CXC chemokines. CXCL2 mobilizes cells by interacting with a cell surface chemokine receptor called CXCR2.

Plerixafor, sold under the brand name Mozobil, is an immunostimulant used to mobilize hematopoietic stem cells in cancer patients into the bloodstream. The stem cells are then extracted from the blood and transplanted back to the patient. The drug was developed by AnorMED, which was subsequently bought by Genzyme.
Gero Hütter is a German hematologist. Hütter and his medical team transplanted bone marrow deficient in a key HIV receptor to a leukemia patient, Timothy Ray Brown, who was also infected with human immunodeficiency virus (HIV). Subsequently, the patient's circulating HIV dropped to undetectable levels. The case was widely reported in the media, and Hütter was named one of the "Berliners of the year" for 2008 by the Berliner Morgenpost, a Berlin newspaper.
CCR5 receptor antagonists are a class of small molecules that antagonize the CCR5 receptor. The C-C motif chemokine receptor CCR5 is involved in the process by which HIV, the virus that causes AIDS, enters cells. Hence antagonists of this receptor are entry inhibitors and have potential therapeutic applications in the treatment of HIV infections.

XMEN disease is a rare genetic disorder of the immune system that illustrates the role of glycosylation in the function of the immune system. XMEN stands for “X-linked MAGT1 deficiency with increased susceptibility to Epstein–Barr virus (EBV) infection and N-linked glycosylation defect.” The disease is characterized by CD4 lymphopenia, severe chronic viral infections, and defective T-lymphocyte activation. Investigators in the laboratory of Dr. Michael Lenardo, National Institute of Allergy and Infectious Diseases at the National Institutes of Health first described this condition in 2011.
A CXCR4 antagonist is a substance which blocks the CXCR4 receptor and prevent its activation. Blocking the receptor stops the receptor's ligand, CXCL12, from binding which prevents downstream effects. CXCR4 antagonists are especially important for hindering cancer progression because one of the downstream effects initiated by CXCR4 receptor activation is cell movement which helps the spread of cancer, known as metastasis. The CXCR4 receptor has been targeted by antagonistic substances since being identified as a co-receptor in HIV and assisting the development of cancer. Macrocyclic ligands have been utilised as CXCR4 antagonists.
Lenzilumab is a humanized monoclonal antibody that targets colony stimulating factor 2 (CSF2)/granulocyte-macrophage colony stimulating factor (GM-CSF).

Mavorixafor, sold under the brand name Xolremdi, is a medication used for the treatment of WHIM syndrome. It is a CXC chemokine receptor 4 antagonist. It is taken by mouth. It was developed by X4 Pharmaceuticals.









