In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal chain reaction can only be achieved with fissile material. The predominant neutron energy in a system may be typified by either slow neutrons or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives.
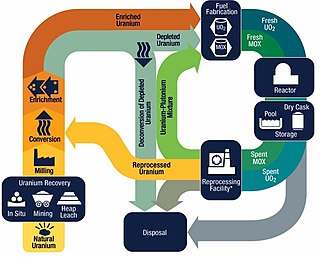
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.

A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. These reactors can be fueled with more-commonly available isotopes of uranium and thorium, such as uranium-238 and thorium-232, as opposed to the rare uranium-235 which is used in conventional reactors. These materials are called fertile materials since they can be bred into fuel by these breeder reactors.

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.

A fast-neutron reactor (FNR) or fast-spectrum reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons, as opposed to slow thermal neutrons used in thermal-neutron reactors. Such a fast reactor needs no neutron moderator, but requires fuel that is relatively rich in fissile material when compared to that required for a thermal-neutron reactor. Around 20 land based fast reactors have been built, accumulating over 400 reactor years of operation globally. The largest was the Superphénix sodium cooled fast reactor in France that was designed to deliver 1,242 MWe. Fast reactors have been studied since the 1950s, as they provide certain advantages over the existing fleet of water-cooled and water-moderated reactors. These are:

The integral fast reactor (IFR), originally the advancedliquid-metal reactor (ALMR), is a design for a nuclear reactor using fast neutrons and no neutron moderator. IFRs can breed more fuel and are distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.
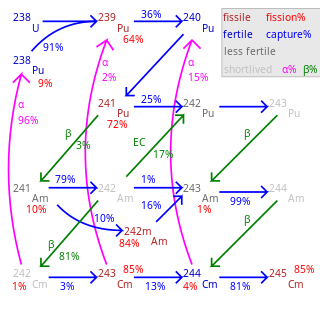
Fertile material is a material that, although not fissile itself, can be converted into a fissile material by neutron absorption.

Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors, along with uranium-235 and uranium-233. Plutonium-239 has a half-life of 24,110 years.
Uranium (92U) is a naturally occurring radioactive element (radioelement) with no stable isotopes. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U. The standard atomic weight of natural uranium is 238.02891(3).
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding 238
U
with neutrons to produce 239
U
, which then underwent beta decay to 239
Np
.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty-two plutonium radioisotopes have been characterized. The most stable are 244Pu with a half-life of 80.8 million years; 242Pu with a half-life of 373,300 years; and 239Pu with a half-life of 24,110 years; and 240Pu with a half-life of 6,560 years. This element also has eight meta states; all have half-lives of less than one second.

The thorium fuel cycle is a nuclear fuel cycle that uses an isotope of thorium, 232
Th
, as the fertile material. In the reactor, 232
Th
is transmuted into the fissile artificial uranium isotope 233
U
which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts of fissile material, which are insufficient to initiate a nuclear chain reaction. Additional fissile material or another neutron source is necessary to initiate the fuel cycle. In a thorium-fuelled reactor, 232
Th
absorbs neutrons to produce 233
U
. This parallels the process in uranium breeder reactors whereby fertile 238
U
absorbs neutrons to form fissile 239
Pu
. Depending on the design of the reactor and fuel cycle, the generated 233
U
either fissions in situ or is chemically separated from the used nuclear fuel and formed into new nuclear fuel.
Plutonium-238 is a radioactive isotope of plutonium that has a half-life of 87.7 years.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started.

Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous.
Uranium-236 is an isotope of uranium that is neither fissile with thermal neutrons, nor very good fertile material, but is generally considered a nuisance and long-lived radioactive waste. It is found in spent nuclear fuel and in the reprocessed uranium made from spent nuclear fuel.
Reactor-grade plutonium (RGPu) is the isotopic grade of plutonium that is found in spent nuclear fuel after the uranium-235 primary fuel that a nuclear power reactor uses has burnt up. The uranium-238 from which most of the plutonium isotopes derive by neutron capture is found along with the U-235 in the low enriched uranium fuel of civilian reactors.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
REMIX-Fuel (REgenerated MIXture of U, Pu oxides) was developed in Russia to simplify the reprocessing process, reuse spent fuel, reduce the consumption of natural uranium and to enable multi-recycling.











