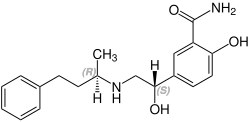| |
| Clinical data | |
|---|---|
| Pronunciation | /ləˈbɛtəlɔːl/ |
| Trade names | Normodyne, Trandate, others |
| Other names | Ibidomide; AH-5158; SCH-19927 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685034 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% (11–86%) [1] [2] [3] |
| Protein binding | 50% [1] [3] |
| Metabolism | Mainly conjugation via glucuronidation [1] [2] [3] |
| Metabolites | • Glucuronide conjugates [2] |
| Elimination half-life | Oral: 6–8 hours [1] [2] [3] IV : 5.52 hours [2] |
| Duration of action | 8–12 hours [1] |
| Excretion | Urine (55–60% as conjugates or unchanged within 24 hours) [1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.401 |
| Chemical and physical data | |
| Formula | C19H24N2O3 |
| Molar mass | 328.412 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Labetalol is a medication used to treat high blood pressure and in long term management of angina. [4] [5] This includes essential hypertension, hypertensive emergencies, and hypertension of pregnancy. [5] In essential hypertension it is generally less preferred than a number of other blood pressure medications. [4] It can be given by mouth or by injection into a vein. [4]
Contents
- Medical uses
- Special populations
- Contraindications
- Side effects
- Common
- Rare
- Pharmacology
- Mechanism of action
- Pharmacokinetics
- Chemistry
- History
- References
Common side effects include low blood pressure with standing, dizziness, feeling tired, and nausea. [4] Serious side effects may include low blood pressure, liver problems, heart failure, and bronchospasm. [4] Use appears safe in the latter part of pregnancy and it is not expected to cause problems during breastfeeding. [5] [6] It works by blocking the activation of β- and α-adrenergic receptors. [4]
Labetalol was patented in 1966 and came into medical use in 1977. [7] It is available as a generic medication. [5] In 2023, it was the 232nd most commonly prescribed medication in the United States, with more than 1 million prescriptions. [8] [9]