The glutamate receptor, metabotropic 1, also known as GRM1, is a human gene which encodes the metabotropic glutamate receptor 1 (mGluR1) protein. [5] [6] [7]
The glutamate receptor, metabotropic 1, also known as GRM1, is a human gene which encodes the metabotropic glutamate receptor 1 (mGluR1) protein. [5] [6] [7]
L-glutamate is the major excitatory neurotransmitter in the central nervous system and activates both ionotropic and metabotropic glutamate receptors. Glutamatergic neurotransmission is involved in most aspects of normal brain function and can be perturbed in many neuropathologic conditions. The metabotropic glutamate receptors are a family of G protein-coupled receptors, that have been divided into 3 groups on the basis of sequence homology, putative signal transduction mechanisms, and pharmacologic properties. Group I, which includes GRM1 alongside GRM5, have been shown to activate phospholipase C. Group II includes GRM2 and GRM3 while Group III includes GRM4, GRM6, GRM7 and GRM8. Group II and III receptors are linked to the inhibition of the cyclic AMP cascade but differ in their agonist selectivities. Alternative splice variants of the GRM1 gene have been described but their full-length nature has not been determined. [5]
A possible connection has been suggested between mGluRs and neuromodulators, as mGluR1 antagonists block adrenergic receptor activation in neurons. [8]
Mice lacking functional glutamate receptor 1 were reported in 1994. By homologous recombination mediated gene targeting those mice became deficient in mGlu receptor 1 protein. The mice did not show any basic anatomical changes in the brain but had impaired cerebellar long-term depression and hippocampal long-term potentiation. In addition they had impaired motor functions, characterized by impaired balance. In the Morris watermaze test, an assay for learning abilities, those mice needed significantly more time to successfully complete the task. [9]
Mutations in the GRM1 gene may contribute to melanoma susceptibility. [10] Antibodies against mGluR1 receptors cause cerebellar ataxia and impair long-term depression (LTDpathies) in the cerebellum. [11]
In addition to the orthosteric site (the site where the endogenous ligand glutamate binds) at least two distinct allosteric binding sites exist on the mGluR1. [12] A respectable number of potent and specific allosteric ligands – predominantly antagonists/inhibitors – has been developed in recent years, although no orthosteric subtype-selective ligands have yet been discovered (2008). [13]


The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.
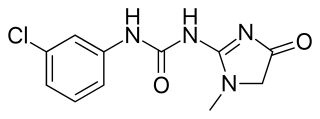
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Metabotropic glutamate receptor 3 (mGluR3) is an inhibitory Gi/G0-coupled G-protein coupled receptor (GPCR) generally localized to presynaptic sites of neurons in classical circuits. However, in higher cortical circuits in primates, mGluR3 are localized post-synaptically, where they strengthen rather than weaken synaptic connectivity. In humans, mGluR3 is encoded by the GRM3 gene. Deficits in mGluR3 signaling have been linked to impaired cognition in humans, and to increased risk of schizophrenia, consistent with their expanding role in cortical evolution.

Metabotropic glutamate receptor 4 is a protein that in humans is encoded by the GRM4 gene.

Metabotropic glutamate receptor 5 is an excitatory Gq-coupled G protein-coupled receptor predominantly expressed on the postsynaptic sites of neurons. In humans, it is encoded by the GRM5 gene.

Metabotropic glutamate receptor 7 is a protein that in humans is encoded by the GRM7 gene.

Metabotropic glutamate receptor 8 is a protein that in humans is encoded by the GRM8 gene.

Eglumetad is a research drug developed by Eli Lilly and Company, which is being investigated for its potential in the treatment of anxiety and drug addiction. It is a glutamate derived compound and its mode of action implies a novel mechanism.
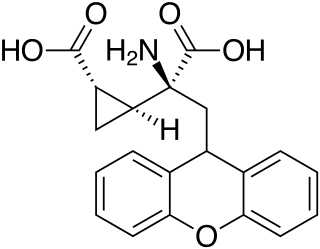
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

Cyclothiazide, sometimes abbreviated CTZ, is a benzothiadiazide (thiazide) diuretic and antihypertensive that was originally introduced in the United States in 1963 by Eli Lilly and was subsequently also marketed in Europe and Japan. Related drugs include diazoxide, hydrochlorothiazide, and chlorothiazide.

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

SIB-1893 is a drug used in scientific research which was one of the first compounds developed that acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It has anticonvulsant and neuroprotective effects, and reduces glutamate release. It has also been found to act as a positive allosteric modulator of mGluR4.

CPCCOEt is a drug used in scientific research, which acts as a non-competitive antagonist at the metabotropic glutamate receptor subtype mGluR1, with high selectivity although only moderate binding affinity. It is used mainly in basic research into the function of the mGluR1 receptor, including the study of behavioural effects in animals including effects on memory and addiction.
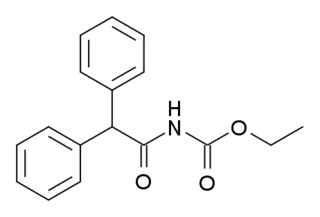
Ro01-6128 is a drug used in scientific research, which acts as a selective positive allosteric modulator for the metabotropic glutamate receptor subtype mGluR1. It was derived by modification of a lead compound found via high-throughput screening, and was further developed to give the improved compound Ro67-4853.

Ro67-4853 is a drug used in scientific research, which acts as a selective positive allosteric modulator for the metabotropic glutamate receptor subtype mGluR1. It was derived by modification of the simpler compound Ro01-6128, and has itself subsequently been used as a lead compound to develop a range of potent and selective mGluR1 positive modulators.

PCCG-4 is a research drug which acts as a selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), with slight selectivity for mGluR2 although not sufficient to distinguish mGluR2 and mGluR3 responses from each other. It is used in research into the function of the group II metabotropic glutamate receptors.

MGS-0039 is a drug that is used in neuroscientific research, which acts as a potent and selective antagonist for group II of the metabotropic glutamate receptors (mGluR2/3). It produces antidepressant and anxiolytic effects in animal studies, and has been shown to boost release of dopamine and serotonin in specific brain areas. Research has suggested this may occur through a similar mechanism as that suggested for the similarly glutamatergic drug ketamine.

AZD 9272 is a drug which acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It was unsuccessful in human trials as an analgesic, but continues to be widely used in research especially as its radiolabelled forms.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.