The adenosine A2B receptor, also known as ADORA2B, is a G-protein coupled adenosine receptor, and also denotes the human adenosine A2b receptor gene which encodes it. [5]
The adenosine A2B receptor, also known as ADORA2B, is a G-protein coupled adenosine receptor, and also denotes the human adenosine A2b receptor gene which encodes it. [5]
This integral membrane protein stimulates adenylate cyclase activity in the presence of adenosine. This protein also interacts with netrin-1, which is involved in axon elongation.
The gene is located near the Smith-Magenis syndrome region on chromosome 17. [5]
Research into selective A2B ligands has lagged somewhat behind the development of ligands for the other three adenosine receptor subtypes, but a number of A2B-selective compounds have now been developed, [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] and research into their potential therapeutic applications is ongoing. [16] [17] [18] [19] [20] [21] [22]

Xanthine is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine.

Adenosine (symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9-glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life on earth. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias.

The adenosine receptors (or P1 receptors) are a class of purinergic G protein-coupled receptors with adenosine as the endogenous ligand. There are four known types of adenosine receptors in humans: A1, A2A, A2B and A3; each is encoded by a different gene.

The Biginelli reaction is a multiple-component chemical reaction that creates 3,4-dihydropyrimidin-2(1H)-ones 4 from ethyl acetoacetate 1, an aryl aldehyde, and urea 3. It is named for the Italian chemist Pietro Biginelli.

The adenosine A1 receptor (A1AR) is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

The adenosine A2A receptor, also known as ADORA2A, is an adenosine receptor, and also denotes the human gene encoding it.

Dopamine receptor D1, also known as DRD1. It is one of the two types of D1-like receptor family — receptors D1 and D5. It is a protein that in humans is encoded by the DRD1 gene.

The adenosine A3 receptor, also known as ADORA3, is an adenosine receptor, but also denotes the human gene encoding it.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Metabotropic glutamate receptor 3 (mGluR3) is an inhibitory Gi/G0-coupled G-protein coupled receptor (GPCR) generally localized to presynaptic sites of neurons in classical circuits. However, in higher cortical circuits in primates, mGluR3 are localized post-synaptically, where they strengthen rather than weaken synaptic connectivity. In humans, mGluR3 is encoded by the GRM3 gene. Deficits in mGluR3 signaling have been linked to impaired cognition in humans, and to increased risk of schizophrenia, consistent with their expanding role in cortical evolution.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

BAY 60–6583 is a selective adenosine A2B receptor agonist. It has been shown to provide protection from ischemia in both the heart and kidney of test animals, and has also been shown to be beneficial in treatment of acute lung and brain injury, as well as claimed anti-aging and anti-obesity effects, showing a range of potential applications for selective A2B agonists.
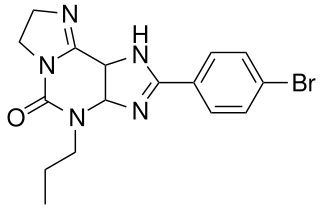
KF-26777 is a drug which acts as a potent and selective antagonist for the adenosine A3 receptor, with sub-nanomolar affinity (A3 Ki=0.2nM) and high selectivity over the other three adenosine receptor subtypes. Simple xanthine derivatives such as caffeine and DPCPX have generally low affinity for the A3 subtype and must be extended by expanding the ring system and adding an aromatic group to give high A3 affinity and selectivity.

PSB-10 is a drug which acts as a selective antagonist for the adenosine A3 receptor (ki value at human A3 receptor is 0.44 nM), with high selectivity over the other three adenosine receptor subtypes (ki values at human A1, A2A and A2B receptors are 4.1, 3.3 and 30 μM). Further pharmacological experiments in a [35S]GTPγS binding assay using hA3-CHO-cells indicated that PSB-10 acts as an inverse agonist (IC50 = 4 nM). It has been shown to produce antiinflammatory effects in animal studies. Simple xanthine derivatives such as caffeine and DPCPX have generally low affinity for the A3 subtype and must be extended by expanding the ring system and adding an aromatic group to give high A3 affinity and selectivity. The affinity towards adenosine A3 subtype was measured against the radioligand PSB-11.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine and active metabolite of mitragynine. It is an analgesic being more potent than morphine.
An adenosine receptor antagonist is a drug which acts as an antagonist of one or more of the adenosine receptors. The best known are xanthines and their derivatives, but there are also non-xanthine representatives

ISAM-140 is a selective non-xanthinic adenosine A2B receptor atagonist. Discovered in 2016, has a Ki of 3.49 nM in A2B receptor and >1000-fold selectivity with respect to the other three adenosine receptor subtypes. It has been shown to help immune system to attack cancer cells in in vitro assays, by rescuing T and NK cell proliferation, cytokine release and TIL infiltration.