Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. Along with mast cells and basophils, they also control mechanisms associated with allergy and asthma. They are granulocytes that develop during hematopoiesis in the bone marrow before migrating into blood, after which they are terminally differentiated and do not multiply.

Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte and how they work is not fully understood. They are responsible for inflammatory reactions during immune response, as well as in the formation of acute and chronic allergic diseases, including anaphylaxis, asthma, atopic dermatitis and hay fever. They also produce compounds that coordinate immune responses, including histamine and serotonin that induce inflammation, and heparin that prevents blood clotting, although there are less than that found in mast cell granules. Mast cells were once thought to be basophils that migrated from the blood into their resident tissues, but they are now known to be different types of cells.
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.

Aspirin-exacerbated respiratory disease (AERD), also called NSAID-exacerbated respiratory disease (N-ERD) or historically aspirin-induced asthma and Samter's Triad, is a long-term disease defined by three simultaneous symptoms: asthma, chronic rhinosinusitis with nasal polyps, and intolerance of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs). Compared to aspirin tolerant patients, AERD patients' asthma and nasal polyps are generally more severe. Reduction or loss of the ability to smell is extremely common, occurring in more than 90% of people with the disease. AERD most commonly begins in early- to mid-adulthood and has no known cure. While NSAID intolerance is a defining feature of AERD, avoidance of NSAIDs does not affect the onset, development or perennial nature of the disease.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.
Interleukin 13 (IL-13) is a protein that in humans is encoded by the IL13 gene. IL-13 was first cloned in 1993 and is located on chromosome 5q31.1 with a length of 1.4kb. It has a mass of 13 kDa and folds into 4 alpha helical bundles. The secondary structural features of IL-13 are similar to that of Interleukin 4 (IL-4); however it only has 25% sequence identity to IL-4 and is capable of IL-4 independent signaling. IL-13 is a cytokine secreted by T helper type 2 (Th2) cells, CD4 cells, natural killer T cell, mast cells, basophils, eosinophils and nuocytes. Interleukin-13 is a central regulator in IgE synthesis, goblet cell hyperplasia, mucus hypersecretion, airway hyperresponsiveness, fibrosis and chitinase up-regulation. It is a mediator of allergic inflammation and different diseases including asthma.

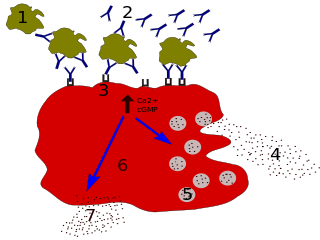
The high-affinity IgE receptor, also known as FcεRI, or Fc epsilon RI, is the high-affinity receptor for the Fc region of immunoglobulin E (IgE), an antibody isotype involved in allergy disorders and parasite immunity. FcεRI is a tetrameric receptor complex that binds Fc portion of the ε heavy chain of IgE. It consists of one alpha, one beta, and two gamma chains connected by two disulfide bridges on mast cells and basophils. It lacks the beta subunit on other cells. It is constitutively expressed on mast cells and basophils and is inducible in eosinophils.

The thromboxane receptor (TP) also known as the prostanoid TP receptor is a protein that in humans is encoded by the TBXA2R gene, The thromboxane receptor is one among the five classes of prostanoid receptors and was the first eicosanoid receptor cloned. The TP receptor derives its name from its preferred endogenous ligand thromboxane A2.
Most of the eicosanoid receptors are integral membrane protein G protein-coupled receptors (GPCRs) that bind and respond to eicosanoid signaling molecules. Eicosanoids are rapidly metabolized to inactive products and therefore are short-lived. Accordingly, the eicosanoid-receptor interaction is typically limited to a local interaction: cells, upon stimulation, metabolize arachidonic acid to an eicosanoid which then binds cognate receptors on either its parent cell or on nearby cells to trigger functional responses within a restricted tissue area, e.g. an inflammatory response to an invading pathogen. In some cases, however, the synthesized eicosanoid travels through the blood to trigger systemic or coordinated tissue responses, e.g. prostaglandin (PG) E2 released locally travels to the hypothalamus to trigger a febrile reaction. An example of a non-GPCR receptor that binds many eicosanoids is the PPAR-γ nuclear receptor.
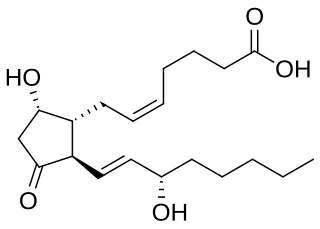
Prostaglandin D2 (or PGD2) is a prostaglandin that binds to the receptor PTGDR (DP1), as well as CRTH2 (DP2). It is a major prostaglandin produced by mast cells – recruits Th2 cells, eosinophils, and basophils. In mammalian organs, large amounts of PGD2 are found only in the brain and in mast cells. It is critical to development of allergic diseases such as asthma. Research carried out in 1989 found PGD2 is the primary mediator of vasodilation (the "niacin flush") after ingestion of niacin (nicotinic acid).
Prostaglandin receptors or prostanoid receptors represent a sub-class of cell surface membrane receptors that are regarded as the primary receptors for one or more of the classical, naturally occurring prostanoids viz., prostaglandin D2,, PGE2, PGF2alpha, prostacyclin (PGI2), thromboxane A2 (TXA2), and PGH2. They are named based on the prostanoid to which they preferentially bind and respond, e.g. the receptor responsive to PGI2 at lower concentrations than any other prostanoid is named the Prostacyclin receptor (IP). One exception to this rule is the receptor for thromboxane A2 (TP) which binds and responds to PGH2 and TXA2 equally well.

The prostaglandin D2 receptor 1 (DP1), a G protein-coupled receptor encoded by the PTGDR1 gene (also termed PTGDR), is primarily a receptor for prostaglandin D2 (PGD2). The receptor is a member of the prostaglandin receptors belonging to the subfamily A14 of rhodopsin-like receptors. Activation of DP1 by PGD2 or other cognate receptor ligands is associated with a variety of physiological and pathological responses in animal models.

Cysteinyl leukotriene receptor 1, also termed CYSLTR1, is a receptor for cysteinyl leukotrienes (LT). CYSLTR1, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans as well as models of the reactions in other animals.

Prostaglandin E2 receptor 2, also known as EP2, is a prostaglandin receptor for prostaglandin E2 (PGE2) encoded by the human gene PTGER2: it is one of four identified EP receptors, the others being EP1, EP3, and EP4, which bind with and mediate cellular responses to PGE2 and also, but with lesser affinity and responsiveness, certain other prostanoids (see Prostaglandin receptors). EP has been implicated in various physiological and pathological responses.

Prostaglandin EP3 receptor (53kDa), also known as EP3, is a prostaglandin receptor for prostaglandin E2 (PGE2) encoded by the human gene PTGER3; it is one of four identified EP receptors, the others being EP1, EP2, and EP4, all of which bind with and mediate cellular responses to PGE2 and also, but generally with lesser affinity and responsiveness, certain other prostanoids (see Prostaglandin receptors). EP has been implicated in various physiological and pathological responses.

The Prostacyclin receptor, also termed the prostaglandin I2 receptor or just IP, is a receptor belonging to the prostaglandin (PG) group of receptors. IP binds to and mediates the biological actions of prostacyclin (also termed Prostaglandin I2, PGI2, or when used as a drug, epoprostenol). IP is encoded in humans by the PTGIR gene. While possessing many functions as defined in animal model studies, the major clinical relevancy of IP is as a powerful vasodilator: stimulators of IP are used to treat severe and even life-threatening diseases involving pathological vasoconstriction.

Ramatroban (INN) is a thromboxane receptor antagonist.

Setipiprant (INN; developmental code names ACT-129968, KYTH-105) is an investigational drug developed for the treatment of asthma and scalp hair loss. It was originally developed by Actelion and acts as a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2). The drug is being developed as a novel treatment for male pattern baldness by Allergan.
The prostaglandin D2 (PGD2) receptors are G protein-coupled receptors that bind and are activated by prostaglandin D2. Also known as PTGDR or DP receptors, they are important for various functions of the nervous system and inflammation. They include the following proteins:
Fevipiprant (INN; code name QAW039) is a drug of the piprant class that was being developed by Novartis. It is a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2 or CRTh2).