
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. During the clotting process, prothrombin is proteolytically cleaved by the prothrombinase enzyme complex to form thrombin. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.
Angiostatin is a naturally occurring protein found in several animal species, including humans. It is an endogenous angiogenesis inhibitor. Clinical trials have been undertaken for its use in anticancer therapy.

The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells form the barrier between vessels and tissue and control the flow of substances and fluid into and out of a tissue.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.
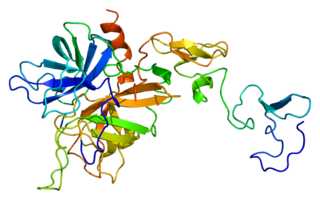
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIV, is a zymogen, that is, an inactive enzyme. The activated form plays an important role in regulating anticoagulation, inflammation, and cell death and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C (APC) performs these operations primarily by proteolytically inactivating proteins Factor Va and Factor VIIIa. APC is classified as a serine protease since it contains a residue of serine in its active site. In humans, protein C is encoded by the PROC gene, which is found on chromosome 2.

Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.
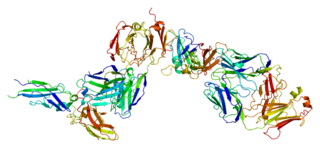
Tissue factor, also called platelet tissue factor, factor III, or CD142, is a protein encoded by the F3 gene, present in subendothelial tissue and leukocytes. Its role in the clotting process is the initiation of thrombin formation from the zymogen prothrombin. Thromboplastin defines the cascade that leads to the activation of factor X—the tissue factor pathway. In doing so, it has replaced the previously named extrinsic pathway in order to eliminate ambiguity.

Cathepsin S is a protein that in humans is encoded by the CTSS gene. Transcript variants utilizing alternative polyadenylation signals exist for this gene.
Kininogens are precursor proteins for kinins, biologically active polypeptides involved in blood coagulation, vasodilation, smooth muscle contraction, inflammatory regulation, and the regulation of the cardiovascular and renal systems.

There are three known thrombin receptors (ThrR), termed PAR1, PAR3 and PAR4.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

Protease activated receptor 3 (PAR-3) also known as coagulation factor II receptor-like 2 (F2RL2) and thrombin receptor-like 2, is a protein that in humans is encoded by the F2RL2 gene.

Protease activated receptor 2 (PAR2) also known as coagulation factor II (thrombin) receptor-like 1 (F2RL1) or G-protein coupled receptor 11 (GPR11) is a protein that in humans is encoded by the F2RL1 gene. PAR2 modulates inflammatory responses, obesity, metabolism, cancers and acts as a sensor for proteolytic enzymes generated during infection. In humans, we can find PAR2 in the stratum granulosum layer of epidermal keratinocytes. Functional PAR2 is also expressed by several immune cells such as eosinophils, neutrophils, monocytes, macrophages, dendritic cells, mast cells and T cells.

Proteinase-activated receptor 1 (PAR1) also known as protease-activated receptor 1 or coagulation factor II (thrombin) receptor is a protein that in humans is encoded by the F2R gene. PAR1 is a G protein-coupled receptor and one of four protease-activated receptors involved in the regulation of thrombotic response. Highly expressed in platelets and endothelial cells, PAR1 plays a key role in mediating the interplay between coagulation and inflammation, which is important in the pathogenesis of inflammatory and fibrotic lung diseases. It is also involved both in disruption and maintenance of endothelial barrier integrity, through interaction with either thrombin or activated protein C, respectively.

Protease-activated receptor 4 (PAR-4), also known as coagulation factor II (thrombin) receptor-like 3, is a protein that in humans is encoded by the F2RL3 gene.
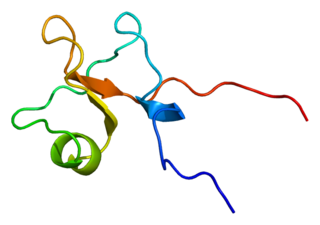
Trefoil factor 3 is a protein that in humans is encoded by the TFF3 gene.

Prader-Willi/Angelman region-1, also known as PWAR1, is an exon of the lncRNA Small nucleolar RNA host gene 14 (SNHG14).
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.

SCH-79797 is a drug which acts as a potent and selective antagonist of the thrombin receptor proteinase activated receptor 1 (PAR1). It has anticoagulant, anticonvulsant and antiinflammatory effects and has been researched as a treatment for heart attack and stroke, though never developed for medical use. It also shows antibiotic actions which are not shared with other PAR1 antagonists such as vorapaxar, so may be mediated through a different target than PAR1.
















