
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Leukotrienes are a family of eicosanoid inflammatory mediators produced in leukocytes by the oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxygenase.

A lipoxin (LX or Lx), an acronym for lipoxygenase interaction product, is a bioactive autacoid metabolite of arachidonic acid made by various cell types. They are categorized as nonclassic eicosanoids and members of the specialized pro-resolving mediators (SPMs) family of polyunsaturated fatty acid (PUFA) metabolites. Like other SPMs, LXs form during, and then act to resolve, inflammatory responses. Initially, two lipoxins were identified, lipoxin A4 (LXA4) and LXB4, but more recent studies have identified epimers of these two LXs: the epi-lipoxins, 15-epi-LXA4 and 15-epi-LXB4 respectively.

Sebacic acid is a naturally occurring dicarboxylic acid with the chemical formula HO2C(CH2)8CO2H. It is a white flake or powdered solid. Sebaceus is Latin for tallow candle, sebum is Latin for tallow, and refers to its use in the manufacture of candles. Sebacic acid is a derivative of castor oil.
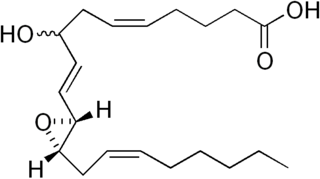
Hepoxilins (Hx) are a set of epoxyalcohol metabolites of polyunsaturated fatty acids (PUFA), i.e. they possess both an epoxide and an alcohol residue. HxA3, HxB3, and their non-enzymatically formed isomers are nonclassic eicosanoid derived from acid the (PUFA), arachidonic acid. A second group of less well studied hepoxilins, HxA4, HxB4, and their non-enzymatically formed isomers are nonclassical eicosanoids derived from the PUFA, eicosapentaenoic acid. Recently, 14,15-HxA3 and 14,15-HxB3 have been defined as arachidonic acid derivatives that are produced by a different metabolic pathway than HxA3, HxB3, HxA4, or HxB4 and differ from the aforementioned hepoxilins in the positions of their hydroxyl and epoxide residues. Finally, hepoxilin-like products of two other PUFAs, docosahexaenoic acid and linoleic acid, have been described. All of these epoxyalcohol metabolites are at least somewhat unstable and are readily enzymatically or non-enzymatically to their corresponding trihydroxy counterparts, the trioxilins (TrX). HxA3 and HxB3, in particular, are being rapidly metabolized to TrXA3, TrXB3, and TrXC3. Hepoxilins have various biological activities in animal models and/or cultured mammalian tissues and cells. The TrX metabolites of HxA3 and HxB3 have less or no activity in most of the systems studied but in some systems retain the activity of their precursor hepoxilins. Based on these studies, it has been proposed that the hepoxilins and trioxilins function in human physiology and pathology by, for example, promoting inflammation responses and dilating arteries to regulate regional blood flow and blood pressure.
Most of the eicosanoid receptors are integral membrane protein G protein-coupled receptors (GPCRs) that bind and respond to eicosanoid signaling molecules. Eicosanoids are rapidly metabolized to inactive products and therefore are short-lived. Accordingly, the eicosanoid-receptor interaction is typically limited to a local interaction: cells, upon stimulation, metabolize arachidonic acid to an eicosanoid which then binds cognate receptors on either its parent cell or on nearby cells to trigger functional responses within a restricted tissue area, e.g. an inflammatory response to an invading pathogen. In some cases, however, the synthesized eicosanoid travels through the blood to trigger systemic or coordinated tissue responses, e.g. prostaglandin (PG) E2 released locally travels to the hypothalamus to trigger a febrile reaction. An example of a non-GPCR receptor that binds many eicosanoids is the PPAR-γ nuclear receptor.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.

Mead acid is an omega-9 fatty acid, first characterized by James F. Mead. As with some other omega-9 polyunsaturated fatty acids, animals can make Mead acid de novo. Its elevated presence in the blood is an indication of essential fatty acid deficiency. Mead acid is found in large quantities in cartilage.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

ALOX12, also known as arachidonate 12-lipoxygenase, 12-lipoxygenase, 12S-Lipoxygenase, 12-LOX, and 12S-LOX is a lipoxygenase-type enzyme that in humans is encoded by the ALOX12 gene which is located along with other lipoyxgenases on chromosome 17p13.3. ALOX12 is 75 kilodalton protein composed of 663 amino acids.

G-protein coupled receptor 31 also known as 12-(S)-HETE receptor is a protein that in humans is encoded by the GPR31 gene. The human gene is located on chromosome 6q27 and encodes a G-protein coupled receptor protein composed of 319 amino acids.

5-Hydroxyeicosatetraenoic acid (5-HETE, 5(S)-HETE, or 5S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. It is produced by diverse cell types in humans and other animal species. These cells may then metabolize the formed 5(S)-HETE to 5-oxo-eicosatetraenoic acid (5-oxo-ETE), 5(S),15(S)-dihydroxyeicosatetraenoic acid (5(S),15(S)-diHETE), or 5-oxo-15-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE).

CYP4F11 is a protein that in humans is encoded by the CYP4F11 gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This gene is part of a cluster of cytochrome P450 genes on chromosome 19. Another member of this family, CYP4F2, is approximately 16 kb away. Alternatively spliced transcript variants encoding the same protein have been found for this gene.
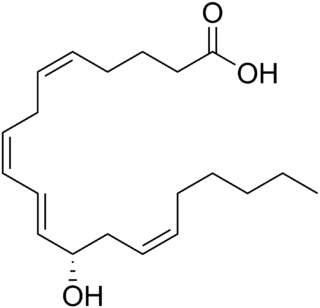
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.
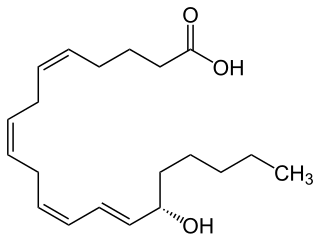
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
Eoxins are proposed to be a family of proinflammatory eicosanoids. They are produced by human eosinophils, mast cells, the L1236 Reed–Sternberg cell line derived from Hodgkin's lymphoma, and certain other tissues. These cells produce the eoxins by initially metabolizing arachidonic acid, an omega-6 (ω-6) fatty acid, via any enzyme possessing 15-lipoxygenase activity. The product of this initial metabolic step, 15(S)-hydroperoxyeicosatetraenoic acid, is then converted to a series of eoxins by the same enzymes that metabolize the 5-lipoxygenase product of arachidonic acid metabolism, i.e. 5-Hydroperoxy-eicosatetraenoic acid to a series of leukotrienes. That is, the eoxins are 14,15-disubstituted analogs of the 5,6-disubstituted leukotrienes.

12-Hydroxyheptadecatrienoic acid (also termed 12-HHT, 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid, or 12(S)-HHTrE) is a 17 carbon metabolite of the 20 carbon polyunsaturated fatty acid, arachidonic acid. It was discovered and structurally defined in 1973 by P. Wlodawer, Bengt I. Samuelsson, and M. Hamberg, as a product of arachidonic acid metabolism made by microsomes (i.e. endoplasmic reticulum) isolated from sheep seminal vesicle glands and by intact human platelets. 12-HHT is less ambiguously termed 12-(S)-hydroxy-5Z,8E,10E-heptadecatrienoic acid to indicate the S stereoisomerism of its 12-hydroxyl residue and the Z, E, and E cis-trans isomerism of its three double bonds. The metabolite was for many years thought to be merely a biologically inactive byproduct of prostaglandin synthesis. More recent studies, however, have attached potentially important activity to it.

5-Oxo-eicosatetraenoic acid is a Nonclassic eicosanoid metabolite of arachidonic acid and the most potent naturally occurring member of the 5-HETE family of cell signaling agents. Like other cell signaling agents, 5-oxo-ETE is made by a cell and then feeds back to stimulate its parent cell and/or exits this cell to stimulate nearby cells. 5-Oxo-ETE can stimulate various cell types particularly human leukocytes but possesses its highest potency and power in stimulating the human eosinophil type of leukocyte. It is therefore suggested to be formed during and to be an important contributor to the formation and progression of eosinophil-based allergic reactions; it is also suggested that 5-oxo-ETE contributes to the development of inflammation, cancer cell growth, and other pathological and physiological events.
5-Hydroxyeicosanoid dehydrogenase (5-HEDH) or more formally, nicotinamide adenine dinucleotide phosphate (NADP+)-dependent dehydrogenase, is an enzyme that metabolizes an eicosanoid product of arachidonate 5-lipoxygenase (5-LOX), 5(S)-hydroxy-6S,8Z,11Z,14Z-eicosatetraenoic acid (i.e. 5-(S)-HETE; see 5-HETE) to its 5-keto analog, 5-oxo-eicosatetraenoic acid (i.e. 5-oxo-6S,8Z,11Z,14Z-eicosatetraenoic acid or 5-oxo-ETE). It also acts in the reverse direction, metabolizing 5-oxo-ETE to 5(S)-HETE. Since 5-oxo-ETE is 30-100-fold more potent than 5(S)-HETE in stimulating various cell types, 5-HEDH is regarded as a regulator and promoter of 5(S)HETE's and thereby 5-LOX's influences on cell function. Although 5-HEDH has been evaluated in a wide range of intact cells and in crude microsome preparations, it has not yet been evaluated for its structure, for its gene, of in pure form; furthermore, most studies on it have been conducted in human tissues.
Cytochrome P450 omega hydroxylases, also termed cytochrome P450 ω-hydroxylases, CYP450 omega hydroxylases, CYP450 ω-hydroxylases, CYP omega hydroxylase, CYP ω-hydroxylases, fatty acid omega hydroxylases, cytochrome P450 monooxygenases, and fatty acid monooxygenases, are a set of cytochrome P450-containing enzymes that catalyze the addition of a hydroxyl residue to a fatty acid substrate. The CYP omega hydroxylases are often referred to as monoxygenases; however, the monooxygenases are CYP450 enzymes that add a hydroxyl group to a wide range of xenobiotic and naturally occurring endobiotic substrates, most of which are not fatty acids. The CYP450 omega hydroxylases are accordingly better viewed as a subset of monooxygenases that have the ability to hydroxylate fatty acids. While once regarded as functioning mainly in the catabolism of dietary fatty acids, the omega oxygenases are now considered critical in the production or break-down of fatty acid-derived mediators which are made by cells and act within their cells of origin as autocrine signaling agents or on nearby cells as paracrine signaling agents to regulate various functions such as blood pressure control and inflammation.
















